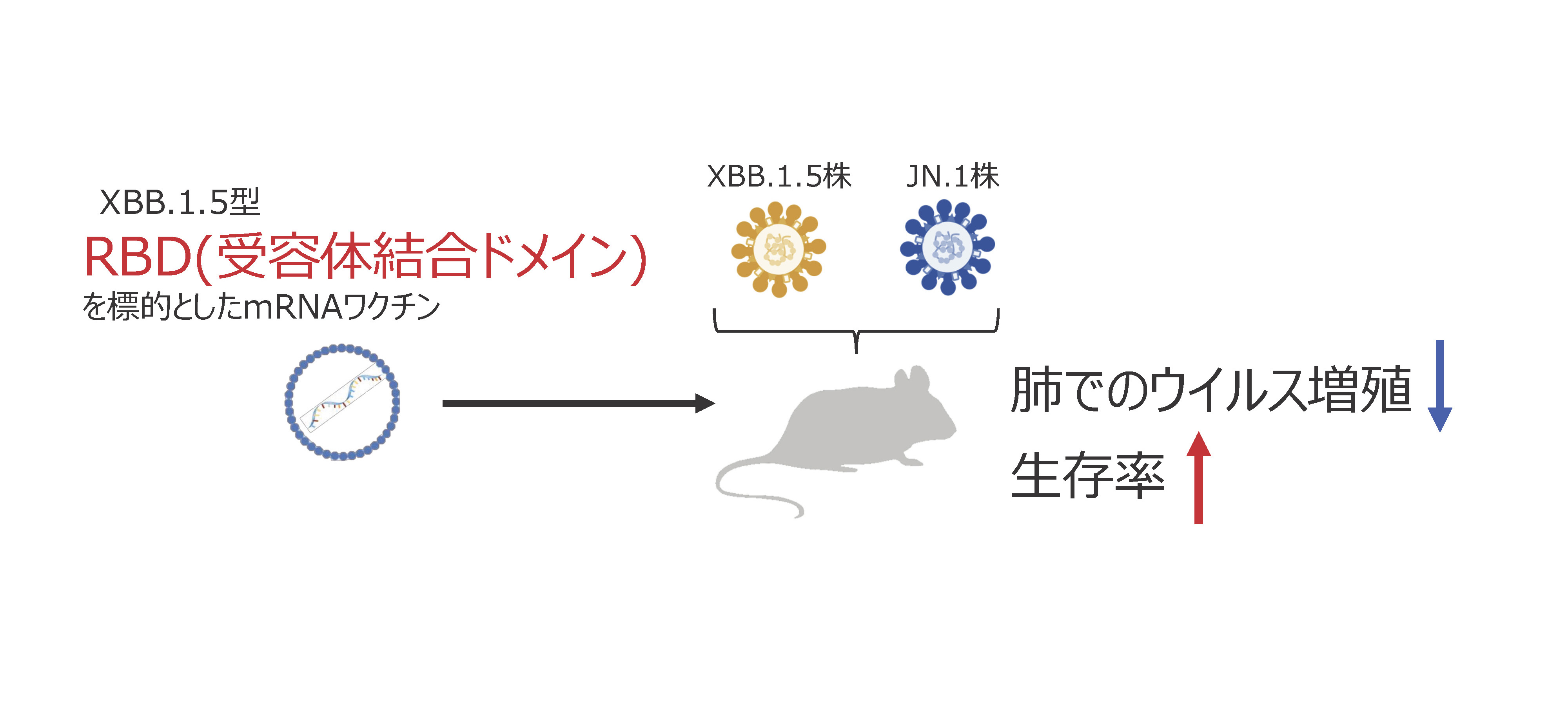
2025-06-13 京都大学
。<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-06-13
- https://www.kyoto-u.ac.jp/sites/default/files/2025-06/web_2506_Nihira-d58984eb07cd3b44bc0514789607ed41.pdf
- https://www.jacionline.org/article/S0091-6749(25)00508-1/fulltext
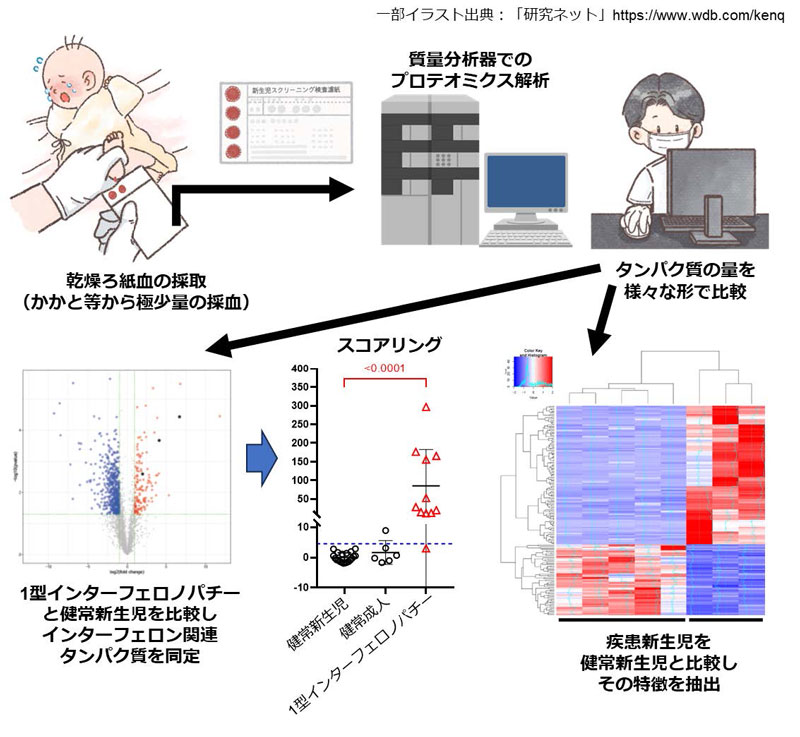
乾燥血液スポットプロテオームから、I型インターフェロンパチー新生児における不顕性インターフェロンシグネチャーが同定される Dried blood spot proteome identifies subclinical interferon signature in neonates with type I interferonopathy
Hiroshi Nihira, MD, PhD ∙ Daisuke Nakajima, PhD ∙ Kazushi Izawa, MD, PhD ∙ … ∙ Ryuta Nishikomori, MD, PhD ∙ Osamu Ohara, PhD ∙ Takahiro Yasumi, MD, PhD
The Journal of Allergy and Clinical Immunology Published:May 2, 2025
DOI:https://doi.org/10.1016/j.jaci.2025.04.025
Abstract
Background
Type I interferonopathy is characterized by aberrant upregulation of type I interferon signaling. The mRNA interferon signature is a useful marker for activation of the interferon pathway and for diagnosis of type I interferonopathy; however, early diagnosis is challenging.
Objective
This study sought to identify the proteomic interferon signature in dried blood spot (DBS) samples. The aim was to evaluate the usefulness of the interferon signature for neonatal screening and to gain insight into presymptomatic state of neonates with inborn errors of immunity (IEIs).
Methods
DBS samples from healthy newborns/adults, patients with type I interferonopathy or other IEIs as well as from neonates with viral infections, including some samples obtained during the presymptomatic neonatal period, were examined by nontargeted proteome analyses. Expression of interferon-stimulated genes (ISGs) was evaluated and a DBS-interferon signature was defined. Differential expression/pathway analysis was also performed.
Results
The ISG products IFIT5, ISG15, and OAS2 were detected. Expression of IFIT5 and ISG15 was upregulated significantly in individuals with type I interferonopathy. We defined the sum of the z scores for these as the DBS-interferon signature, and found that patients with IEIs other than type I interferonopathy, such as chronic granulomatous disease (CGD), also showed significant elevation. Additionally, neonatal samples of type I interferonopathy and CGD patients showed high interferon signatures. Pathway analysis of neonatal CGD samples revealed upregulation of systemic lupus erythematosus–like pathways.
Conclusion
Upregulation of the interferon pathway exists already at birth—not only in neonates with type I interferonopathy but also in other IEIs, including CGD.