2025-06-23 中国科学院(CAS)
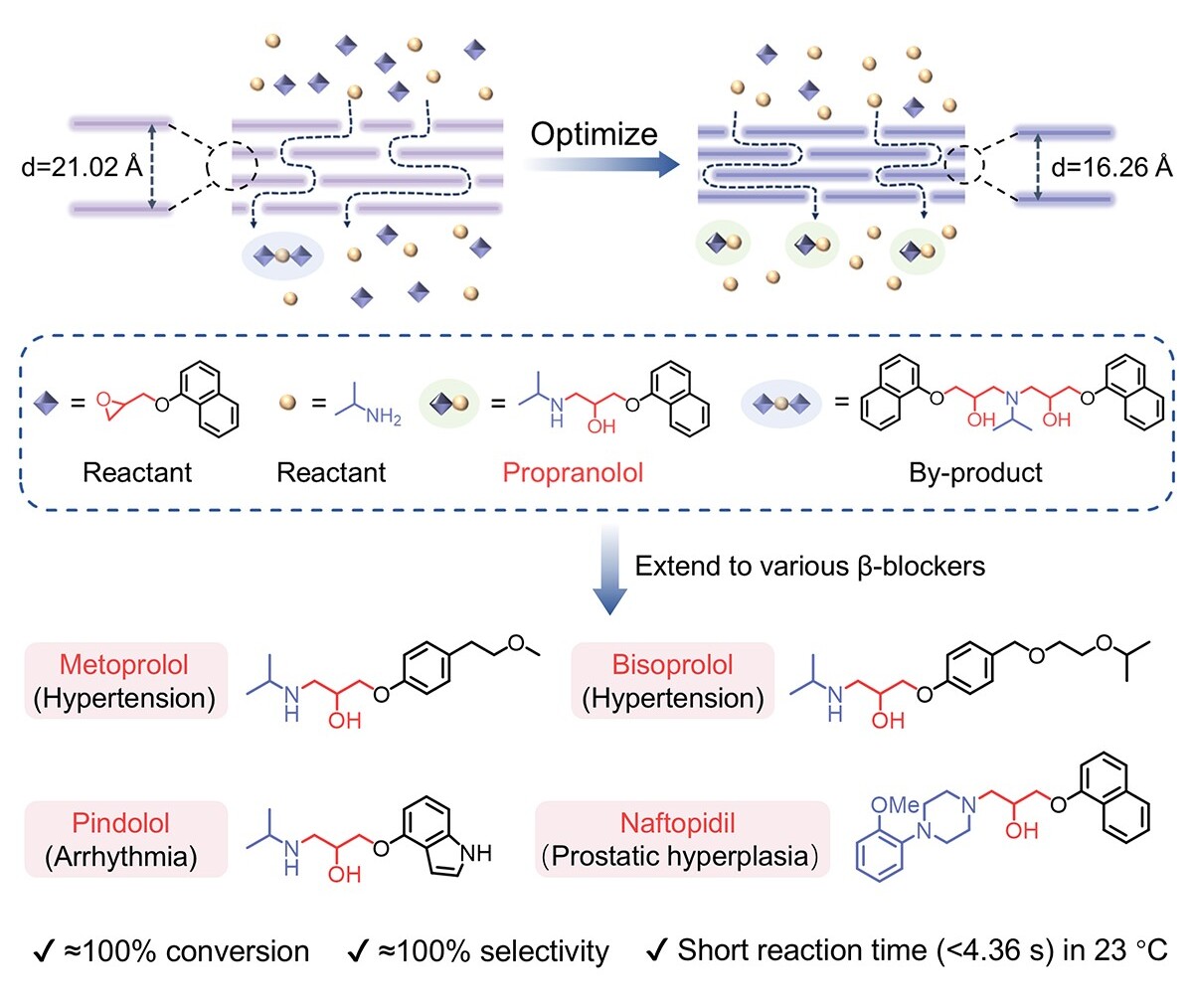 Schematic illustration of the NGO membrane reactor for the synthesis of β-blockers. (Image by ZHANG’s Group)
Schematic illustration of the NGO membrane reactor for the synthesis of β-blockers. (Image by ZHANG’s Group)
<関連情報>
- https://english.cas.cn/newsroom/research_news/chem/202506/t20250617_1045740.shtml
- https://www.cell.com/matter/abstract/S2590-2385(25)00286-3
β遮断薬の温和かつ高効率合成のための膜ナノリアクター Membrane nanoreactors for mild and high-efficiency synthesis of β-blockers
Jiangwei Fu ∙ Xiang Li,,, ∙ Guandi He ∙ … ∙ Daoling Peng ∙ Xiqi Zhang,,, ∙ Lei Jiang
Matter Published:June 20, 2025
DOI:https://doi.org/10.1016/j.matt.2025.102243
Progress and potential
The synthesis of β-blockers often suffers from low efficiency, undesirable by-products and difficulties in separation and purification. In this work, we develop amine-functionalized graphene oxide membrane reactors for synthesis of β-blockers with ≈100% conversion and ≈100% selectivity in less than 4.63 s at 23°C. This performance is achieved through synergistic interlayer spacing tuning and reactant molar ratio optimization under nanoconfinement. The approach might be generalizable to other active pharmaceutical ingredients and membrane materials, offering a scalable, low-energy platform for green pharmaceutical manufacturing and future continuous-flow synthesis technologies.
Highlights
•Membrane nanoreactors for mild and high-efficiency β-blocker synthesis at 23°C
•Synthesis of propranolol with ≈100% conversion and ≈100% selectivity in <4.63 s
•Interlayer confinement shifts the reaction from thermodynamic to kinetic control
•Catalytic platform extended to various β-blockers with high efficiency
Summary
β-blockers, such as propranolol, are widely used in the treatment of cardiovascular diseases. However, current catalytic systems for their synthesis often suffer from low efficiency, poor selectivity, and the need for harsh reaction conditions. Here, amine-functionalized graphene oxide (NGO) membrane nanoreactors were developed for the efficient synthesis of propranolol. By incorporating alkaline catalytic sites and optimizing interlayer spacing and reactant molar ratios, propranolol synthesis was achieved with directional flow, ≈100% conversion, and ≈100% selectivity in less than 4.63 s at 23°C. Density functional theory calculations revealed that adjusting the interlayer spacing of the NGO membrane promoted the reaction from thermodynamic to kinetic control through the confinement effect. The method was successfully extended to the synthesis of metoprolol, bisoprolol, pindolol, and naftopidil, demonstrating high-efficiency flow synthesis for various β-blockers. This work offers a highly efficient, environmentally friendly approach for the synthesis of β-blockers with high conversion and selectivity.


