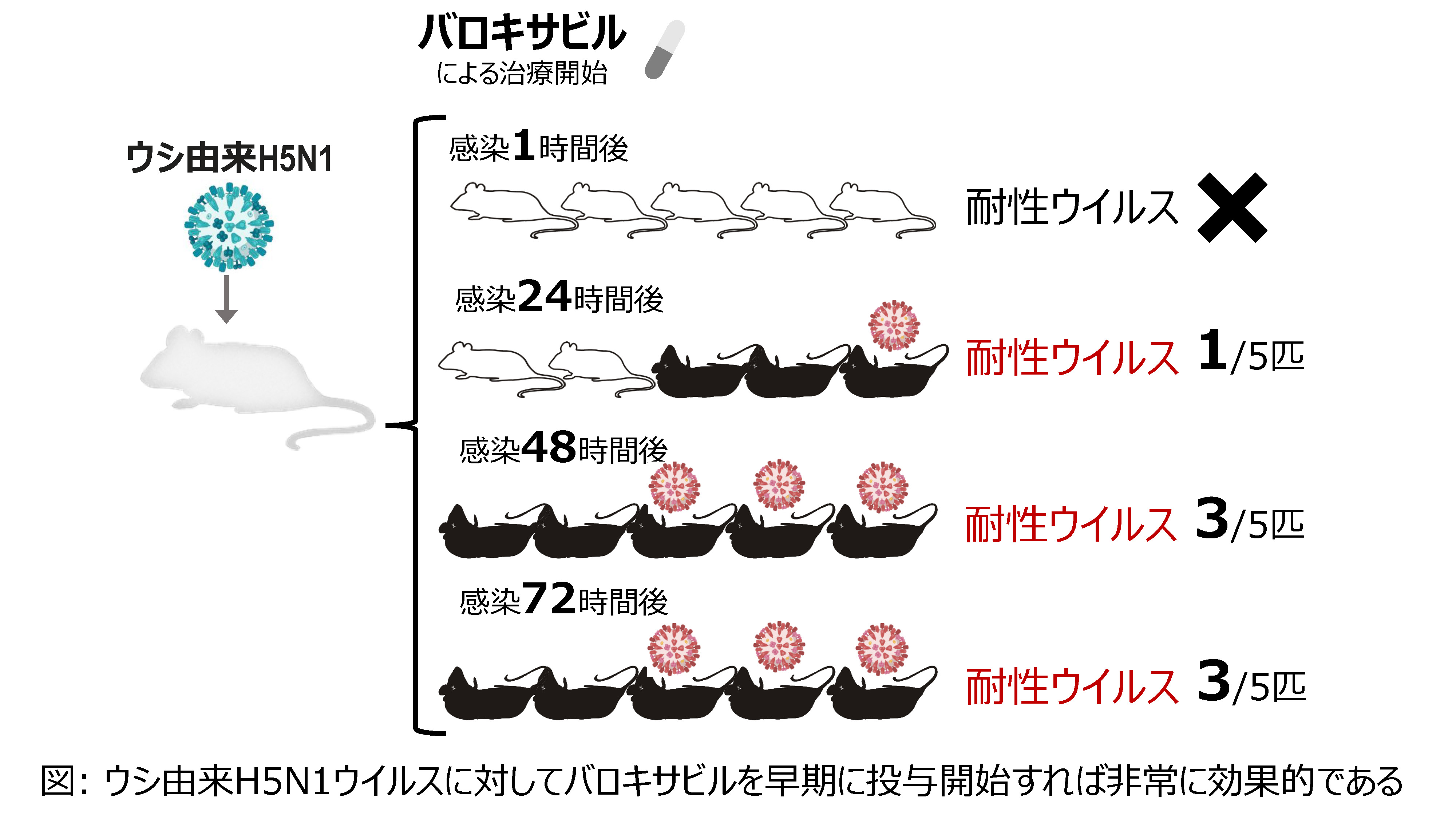
2025-06-24 東京科学大学
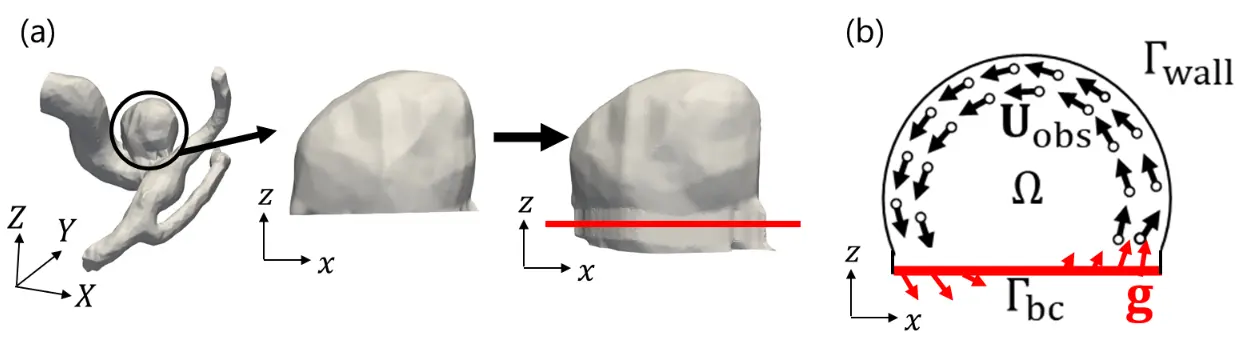
図1. 脳動脈瘤のみを解析対象とした血流のデータ同化解析の問題設定(掲載論文より引用)
<関連情報>
- https://www.isct.ac.jp/ja/news/56mslt9exqjy#top
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=1814&prevId=&key=9ef1769c43c2d23729c6b0555489ab93.pdf
- https://www.sciencedirect.com/science/article/pii/S0169260725002780
流速の境界制御を伴う変分法を用いた脳動脈瘤内流れのデータ同化のための実用的戦略 A practical strategy for data assimilation of cerebral intra-aneurysmal flows using a variational method with boundary control of velocity
Tsubasa Ichimura, Shigeki Yamada, Yoshiyuki Watanabe, Hiroto Kawano, Satoshi Ii
Computer Methods and Programs in Biomedicine Available online: 19 May 2025
DOI:https://doi.org/10.1016/j.cmpb.2025.108861
Highlights
- A practical strategy for variational DA for personalized intra-aneurysmal flows.
- An inverse problem to estimate velocity profile at an aneurysm neck.
- A model order reduction using Fourier series for temporal change of the velocity.
- A velocity mismatch of approximately 4-7% for synthetic data.
- Superior estimation of the present DA to the standard CFD for 4D flow MRI data.
Abstract
Background and objective
Evaluation of hemodynamics is crucial to predict growth and rupture of cerebral aneurysms. Variational data assimilation (DA) is a powerful tool to characterize patient-specific intra-aneurysmal flows. The DA inversely estimates a boundary condition in fluid equations using personalized flow data; however, its high computational cost in optimization problems makes its use impractical.
Methods
This study proposes a practical strategy for the DA to evaluate patient-specific intra-aneurysmal flows. To estimate personalized flows, a variational DA was combined with computational fluid dynamics (CFD) and four-dimensional flow magnetic resonance imaging (4D flow MRI) for intra-aneurysmal velocity data, and an inverse problem was solved to estimate the spatiotemporal velocity profile at a boundary of the aneurysm neck. To circumvent an ill-posed inverse problem, model order reduction based on a Fourier series expansion was used to describe temporal changes in state variables.
Results
In numerical validation using synthetic data from the CFD, the present DA achieved excellent agreement with the CFD as ground truth, with velocity mismatch within the 4%-7% range. In flow estimations for three patient-specific datasets, the proposed DA shows the velocity mismatch within the 35%-63% range, which is less than half that of the CFD using main vessel branches, and would mitigate unphysical velocity distributions in the 4D flow MRI.
Conclusions
By focusing only on the intra-aneurysmal region, the present strategy based on the DA provides an attractive way to evaluate personalized flows in aneurysms with greater reliability than conventional CFD and better efficiency than existing DA approaches.