2025-06-30 マウントサイナイ医療システム (MSHS)
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/mount-sinai-researcher-publishes-landmark-studies-advancing-treatment-for-rare-and-aggressive-lymphoma
- https://www.sciencedirect.com/science/article/pii/S000649712500936X
- https://www.nature.com/articles/s41408-025-01236-6
- https://ascopubs.org/doi/full/10.1200/JCO.24.00033
リヒター変容の診断、評価、研究に関する国際的コンセンサス・ステートメント:ERIC勧告 International Consensus Statement on Diagnosis, Evaluation, and Research of Richter Transformation: the ERIC Recommendations
Adam S Kittai,Monia Marchetti,Othman Al-Sawaf,Ohad Benjamini,Alexey V Danilov,Matthew S. Davids,Barbara F. Eichhorst,Toby A. Eyre,Anna Maria Frustaci,Michael J Hallek,Paul Joseph Hampel,Yair Herishanu,Rodney John Hicks,Arnon P. Kater,Rebecca L. King,José-Ignacio Ignacio Martín-Subero,Carolyn Owen,Erin M. Parry,Maurilio Ponzoni,Davide Rossi,Tanya Siddiqi,Stephan Stilgenbauer,Constantine S. Tam,Elisa ten Hacken,Philip A Thompson,William G. Wierda,Gianluca Gaidano,Jennifer A. Woyach,Paolo Ghia
blood Published:April 16, 2025
DOI:https://doi.org/10.1182/blood.2024028064
Key Points
- Investigation of RT tissue is required for appropriate diagnosis and to understand the pathobiology of this poor-risk lymphoma subtype.
- Due to the poor prognosis of RT with conventional chemoimmunotherapy, participation in clinical trials should be prioritized.
Richter transformation (RT) is defined as an aggressive lymphoma emerging in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). Despite novel therapeutics developed in CLL, RT is associated with poor outcomes. In light of recent progress regarding the diagnostic procedures and therapeutic concepts of RT, an international group of experts, under the coordination of the European Research Initiative on CLL (ERIC), has developed consensus recommendations for clinical procedures and future research on this disease. Patients with RT typically present with a rapid clinical decline, worsening B-symptoms, elevated LDH, and/or rapidly enlarging lymphadenopathy. Workup should include a PET-CT for patients with suspected RT. An excisional biopsy should be taken from an accessible lesion, preferably with the highest FDG avidity, and analyzed for the presence of aggressive lymphoma. The molecular relationship to the original CLL clone(s) should be defined. As no effective standard treatment for RT exists, patients should be treated in a clinical trial. Response of both RT and CLL should be assessed at an early time point, and survival endpoints should be prioritized in trial design. We hope that these recommendations can help to harmonize clinical and translational research and improve outcomes for patients with RT.
CLLに対して化学免疫療法を受けたことのないリヒター変容患者の転帰 Outcomes of patients with Richter transformation who received no prior chemoimmunotherapy for their CLL
Adam S. Kittai,Ying Huang,Sarah Miller,John N. Allan,Seema A. Bhat,David A. Bond,Danielle M. Brander,John C. Byrd,Julio C. Chavez,Elise Chong,Matthew S. Davids,Alexey V. Danilov,Wei Ding,Mark R. Dowling,Kaitlyn Dvorak-Kornaus,Hannah Freedman,Paul J. Hampel,Carrie Ho,Steven R. Hwang,Prioty Islam,Nikita Malakhov,Matthew Matasar,Cecelia Miller,Zulfa Omer,… Jennifer A. Woyach
Blood Cancer Journal Published:20 February 2025
DOI:https://doi.org/10.1038/s41408-025-01236-6
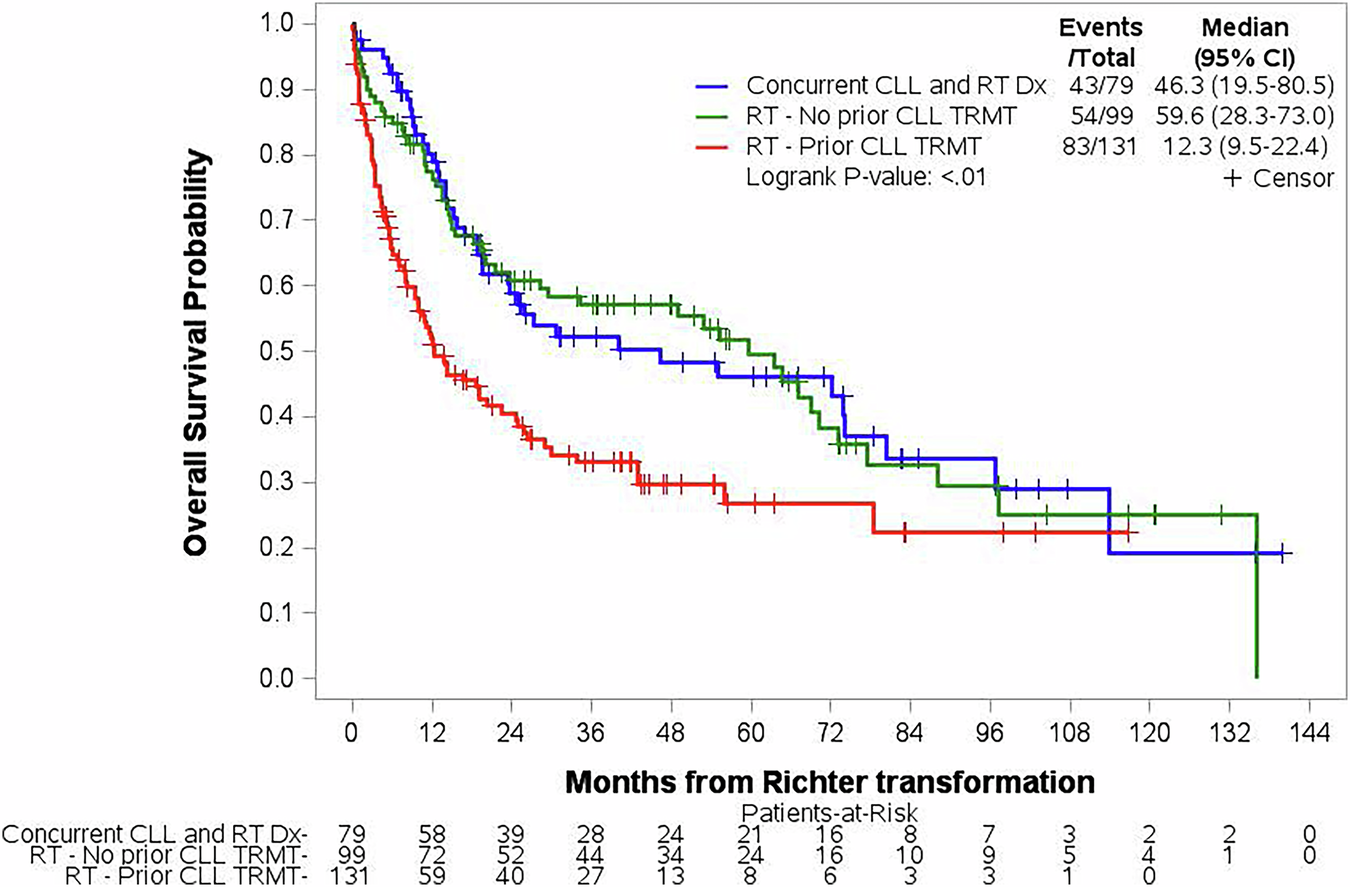
Richter transformation (RT) is an infrequent but consequential event that can occur at any time for patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). RT is defined by the development of an aggressive lymphoma, most often diffuse large B-cell Lymphoma (DLBCL) [1]. Targeted therapies such as Bruton Tyrosine Kinase inhibitors (BTKi) and B-cell leukemia/lymphoma 2 inhibitors (BCL2i) have improved survival for patients with CLL, but not for patients with RT [2, 3].
There have been multiple studies looking at RT that developed after patients received both chemoimmunotherapy (CIT) and targeted therapies [2,3,4,5], but no study has focused on RT that has developed without prior CIT. Given the change in standard of care from the use of CIT to targeted agents, it is important to evaluate RT that develops in patients who have only received targeted therapies. Therefore, we sought to determine survival rates and variables that predict survival in patients who developed RT without prior CIT exposure for CLL.
リヒター転移に対する抗CD19キメラ抗原受容体T細胞療法: 国際多施設共同レトロスペクティブ研究 Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy for Richter Transformation: An International, Multicenter, Retrospective Study
Adam S. Kittai, MD, David Bond, MD, Ying Huang, MA, MS, Seema A. Bhat, MD, Emily Blyth, B.Med(Hons), PhD, FRACP, FRCPA, John C. Byrd, MD, Julio C. Chavez, MD, MS, …
Journal of Clinical Oncology Published:March 29, 2024
DOI:https://doi.org/10.1200/JCO.24.00033
Abstract
Purpose
Outcomes for Richter transformation (RT) are poor with current therapies. The efficacy and safety of anti-CD19 chimeric antigen receptor T-cell therapy (CAR-T) for RT are not established.
Methods
We performed an international multicenter retrospective study of patients with RT who received CAR-T. Patient, disease, and treatment characteristics were summarized using descriptive statistics, and modeling analyses were used to determine association with progression-free survival (PFS) and overall survival (OS). PFS and OS were estimated from the date of CAR-T infusion.
Results
Sixty-nine patients were identified. The median age at CAR-T infusion was 64 years (range, 27-80). Patients had a median of four (range, 1-15) previous lines of therapy for CLL and/or RT, including previous Bruton tyrosine kinase inhibitor and/or BCL2 inhibitor therapy in 58 (84%) patients. The CAR-T product administered was axicabtagene ciloleucel in 44 patients (64%), tisagenlecleucel in 17 patients (25%), lisocabtagene maraleucel in seven patients (10%), and brexucabtagene autoleucel in one patient (1%). Eleven patients (16%) and 25 patients (37%) experienced grade ≥3 cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome, respectively. The overall response rate was 63%, with 46% attaining a complete response (CR). After a median follow-up of 24 months, the median PFS was 4.7 months (95% CI, 2.0 to 6.9); the 2-year PFS was 29% (95% CI, 18 to 41). The median OS was 8.5 months (95% CI, 5.1 to 25.4); the 2-year OS was 38% (95% CI, 26 to 50). The median duration of response was 27.6 months (95% CI, 14.5 to not reached) for patients achieving CR.
Conclusion
CAR-T demonstrates clinical efficacy for patients with RT.