2025-07-17 カリフォルニア大学サンディエゴ校 (UCSD)
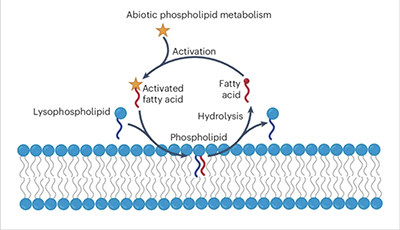
The chemical process begins with the activation of a fatty acid, which then spontaneously couples with a lysophospholipid (LP) to generate a phospholipid. The chemical linkage formed is transient and eventually the phospholipid returns to its LP and fatty acid precursors. The stars represent chemical energy activating the fatty acid. (cr: Neal Devaraj lab / UC San Diego)
<関連情報>
- https://today.ucsd.edu/story/origins-of-life-cellular-metabolism
- https://www.nature.com/articles/s41557-025-01829-5
生物由来の脂質代謝が人工細胞の膜可塑性を可能にする Abiotic lipid metabolism enables membrane plasticity in artificial cells
Alessandro Fracassi,Andrés Seoane,Roberto J. Brea,Hong-Guen Lee,Alexander Harjung & Neal K. Devaraj
Nature Chemistry Published:22 May 2025
DOI:https://doi.org/10.1038/s41557-025-01829-5
Abstract
The plasticity of living cell membranes relies on complex metabolic networks fueled by cellular energy. These metabolic processes exert direct control over membrane properties such as lipid composition and morphological plasticity, which are essential for cellular functions. Despite notable progress in the development of artificial systems mimicking natural membranes, the realization of synthetic membranes capable of sustaining metabolic cycles remains a challenge. Here we present an abiotic phospholipid metabolic network that generates and maintains dynamic artificial cell membranes. Chemical coupling agents drive the in situ synthesis of transiently stable non-canonical phospholipids, leading to the formation and maintenance of phospholipid membranes. We find that phospholipid metabolic cycles can drive lipid self-selection, favouring the enrichment of specific lipid species. Moreover, we demonstrate that controlling lipid metabolism can induce reversible membrane phase transitions, facilitating lipid mixing between distinct populations of artificial membranes. Our work demonstrates that a simple lipid metabolic network can drive dynamic behaviour in artificial membranes, offering insights into mechanisms for engineering functional synthetic compartments.

