2025-07-25 京都大学
<関連情報>
- https://www.kyoto-u.ac.jp/ja/research-news/2025-07-25-2
- https://www.kyoto-u.ac.jp/sites/default/files/2025-07/web_2507_Jo-d89ac36009a053e2496c87bec97bf49d.pdf
- https://onlinelibrary.wiley.com/doi/10.1111/bjh.20266
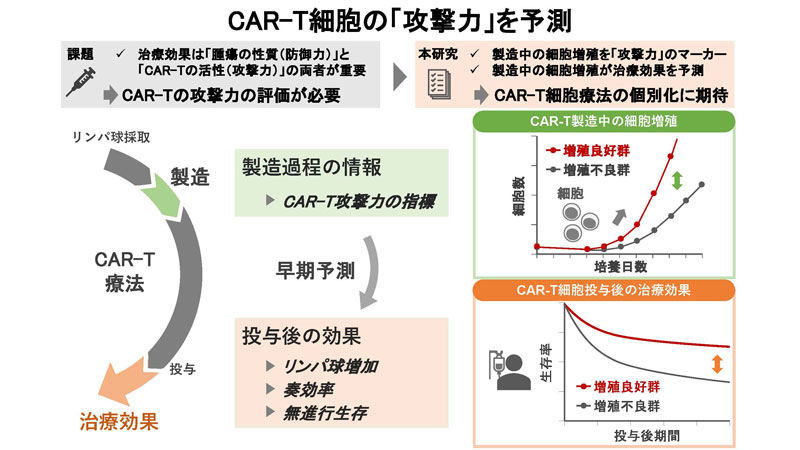
製造中のex vivo細胞増殖の一貫性が、CAR-T細胞療法後の良好な転帰を予測する Consistent ex vivo cell proliferation during manufacturing predicts favourable outcomes post-CAR-T-cell therapy
Tomoyasu Jo, Yasuyuki Arai, Tomoshige Shimizu, Toshio Kitawaki, Takashi Sakamoto, Chisaki Mizumoto, Junya Kanda, Momoko Nishikori, Kohei Yamashita, Miki Nagao, Akifumi Takaori-Kondo
British Journal of Haematology Published: 09 July 2025
DOI:https://doi.org/10.1111/bjh.20266
Summary
Efficacy of chimeric antigen receptor (CAR) T-cell therapy hinges on CAR-T potency, as well as on tumour traits and disease status. Potency assessment currently relies on post-infusion in vivo growth; therefore, manufacturing metrics could provide early potency predictions, enabling potency-guided strategies. To assess the impact of ex vivo cell growth during manufacturing on clinical outcomes, we analysed diffuse large B-cell lymphoma patients treated with tisagenlecleucel, merging clinical records with manufacturing parameters. Of 75 cases, 21 (28%) showed poor growth, indicated by any decrease in cell number during manufacturing. Good growth correlated with significantly better overall response rate (85.2% vs. 52.4%; p = 0.006), with enhanced lymphocyte increase in peripheral blood after infusion. Multivariate analysis revealed that this group had significantly higher progression-free survival (PFS) (adjusted hazard ratio [aHR]: 0.377; 95% confidence interval [CI]: 0.180–0.789; p = 0.010) and overall survival (OS) (aHR: 0.191; 95% CI: 0.071–0.510; p = 0.001), with lower cumulative incidence of disease progression (aHR: 0.412; 95% CI: 0.198–0.858; p = 0.018), compared to the poor growth group, even after adjusting for clinical factors, bridging therapies performed between apheresis and infusion and disease status at infusion. Our findings suggest that good cell growth during manufacturing predicts favourable post-CAR-T outcomes. Identifying clinical factors that influence manufacturing parameters could improve post-CAR-T outcomes.