2025-07-30 トロント大学
<関連情報>
- https://www.utoronto.ca/news/disrupted-sleep-damages-blood-vessels-brain-and-may-increase-dementia-risk-study
- https://academic.oup.com/brain/advance-article/doi/10.1093/brain/awaf161/8189044?login=false
アルツハイマー病の有無にかかわらず成人における睡眠、ペリサイトサブタイプと認知機能低下 Sleep, pericyte subtypes and cognitive decline in adults with and without Alzheimer’s disease
Mahnoor Hamid , Trishna Saha Detroja , Shreejoy J Tripathy , Vilas Menon , Jishu Xu , Lei Yu , Yanling Wang , Aron S Buchman , David A Bennett , Philip L De Jager …
Brain Published:14 July 2025
DOI:https://doi.org/10.1093/brain/awaf161
Abstract
Sleep fragmentation is common in older adults and is associated with cognitive impairment and dementia, as well as key histopathological correlates of dementia, including small vessel disease and cerebral infarcts. Vascular and blood–brain barrier dysfunction are thought to contribute to cognitive decline and dementia. Pericytes, a key vascular cell type, may play a key role.
In model organisms, sleep disruption is associated with pericyte dysfunction and blood–brain barrier breakdown. Recent advances in single-nucleus RNA sequencing (snRNAseq) technology have identified two transcriptionally distinct subtypes of pericytes: extracellular matrix protein-expressing M-pericytes and solute carrier-expressing T-pericytes. However, the relationship between sleep, pericyte biology and cognition in humans remains unclear.
We tested the hypothesis that differences in the composition of brain pericyte subpopulations, as inferred from marker gene expression, may link sleep fragmentation and cognitive decline. We leveraged two published human brain snRNAseq datasets to identify specific marker genes for M- and T-type pericytes. We then used post-mortem bulk RNAseq data from the dorsolateral prefrontal cortex (n = 1092) and lateral orbitofrontal cortex (n = 495) to quantify expression of these marker genes in older adults in two longitudinal cohort studies: the Religious Orders Study and Rush Memory and Aging Project. We derived trajectories of global cognitive function from participants’ ante-mortem annual cognitive assessments, while sleep fragmentation was derived from ante-mortem wrist-actigraphy recordings from a subset of 572 participants. We used multivariate linear regression to relate pericyte marker gene expression to sleep fragmentation and cognitive decline in the decade preceding death.
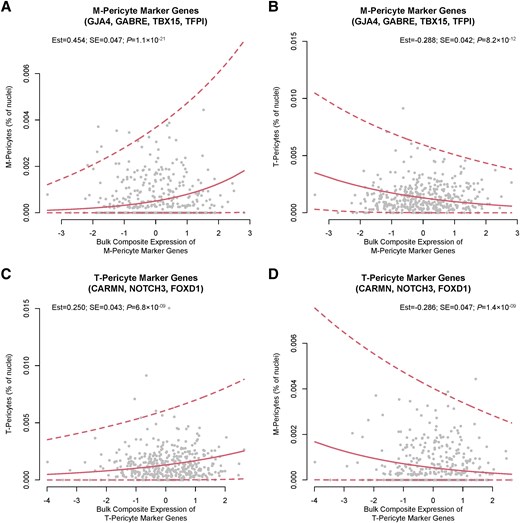
In the dorsolateral prefrontal cortex, greater average sleep fragmentation was associated with greater expression of M-pericyte marker genes [estimate = +3.65 × 10-1, standard error (SE) = 1.61 × 10-1, P = 0.024] but not T-pericyte marker genes. Dorsolateral prefrontal cortex expression of M-pericyte (estimate = -8.30 × 10-3, SE = 3.37 × 10-3, P = 0.014) but not T-pericyte marker genes was associated with more rapid cognitive decline in the 10 years prior to death. In the lateral orbitofrontal cortex, greater sleep fragmentation was also associated with greater composite M-pericyte gene expression (estimate = + 4.48 × 10-1, SE = 1.92 × 10-1, P = 0.02), which in turn was associated with faster cognitive decline in the decade preceding death (estimate = -1.30 × 10-2, SE = 4.55 × 10-3, P = 0.0044).
These findings identify a potential role of M-pericytes in linking sleep fragmentation and cognitive trajectories in older adults. Additionally, our findings highlight the importance of vascular mechanisms in linking disrupted sleep to dementia.

