2025-08-20 カロリンスカ研究所(KI)
<関連情報>
- https://news.ki.se/how-mutations-in-bodily-tissues-affect-ageing
- https://www.nature.com/articles/s43587-025-00941-y
- https://www.nature.com/articles/s43587-025-00882-6
骨格筋再生中の誘導性体細胞変異の蓄積が筋力を低下させる Induced somatic mutation accumulation during skeletal muscle regeneration reduces muscle strength
Peter Vrtačnik,Lara G. Merino,Santhilal Subhash,Hafdís T. Helgadóttir,Matthieu Bardin,Fabiana Stefani,Depin Wang,Ping Chen,Irene Franco,Gwladys Revêchon & Maria Eriksson
Nature Aging Published:20 August 2025
DOI:https://doi.org/10.1038/s43587-025-00941-y
Abstract
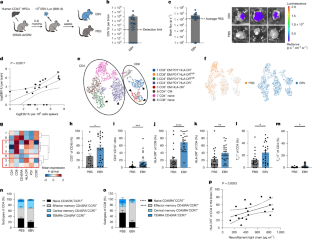
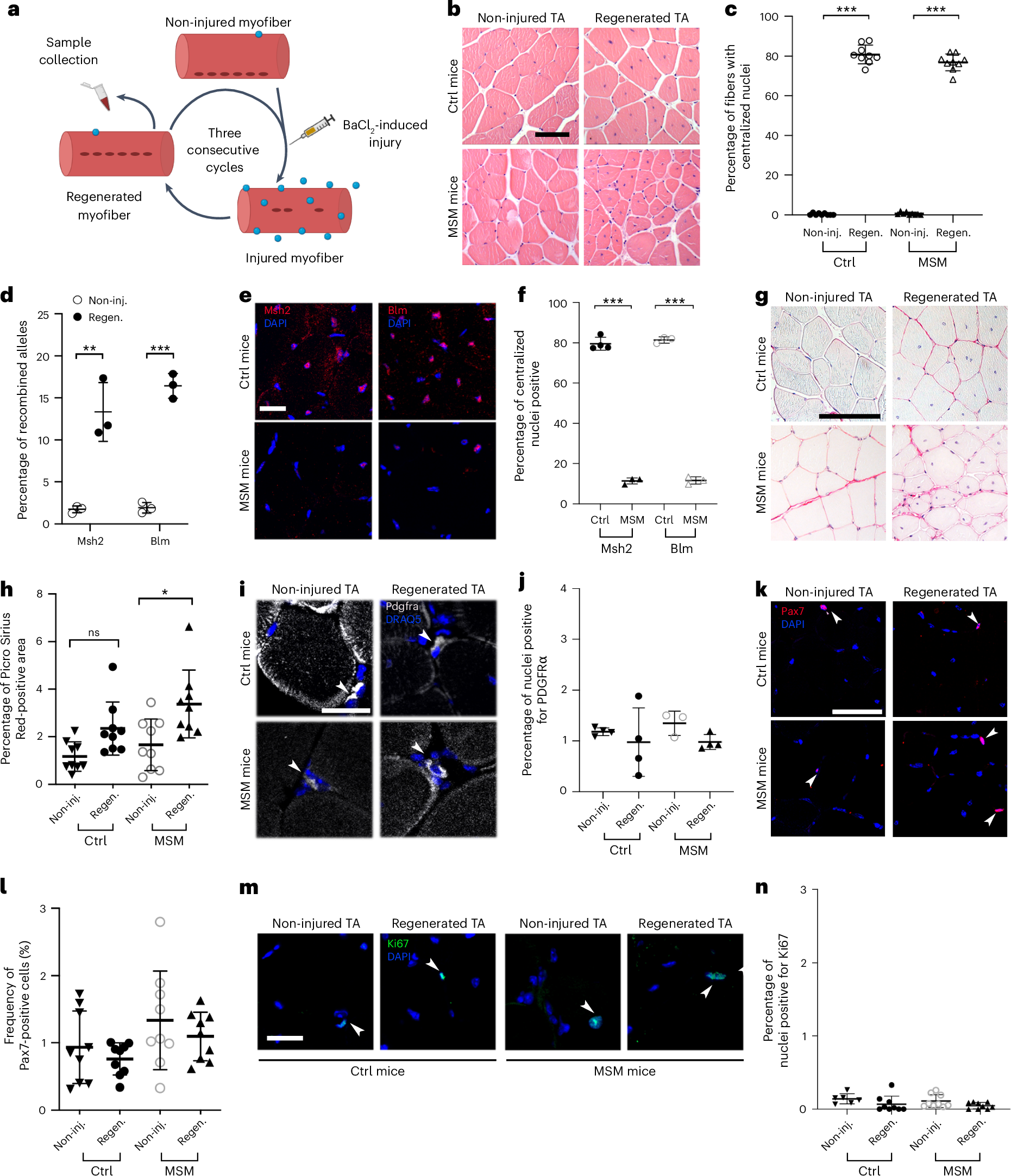
Aging is associated with a progressive decline in tissue function and regenerative capacity, partly due to genomic instability, one of the hallmarks of aging1,2. Genomic instability encompasses DNA damage and the accumulation of somatic mutations in post-zygotic cells, yet the specific impact of these mutations on age-related tissue dysfunction remains poorly understood. To address this, we developed a mouse model in which genomic instability was induced specifically in muscle progenitor cells3 through targeted deletion of the Msh2 (ref. 4) and Blm5 genes. This allowed us to assess how elevated DNA damage and somatic mutations, from single-nucleotide variants (SNVs) to structural variants, affect muscle regeneration following injury. These mice exhibited impaired muscle regeneration, characterized by smaller muscle fibers, reduced muscle mass gain and decreased grip strength. Importantly, similar muscle deficits were observed in a second mouse model where somatic mutations were elevated with less substantial DNA damage. These findings provide evidence that the accumulation of somatic mutations can potentially compromise the function of somatic cells, contributing to the aging phenotype in skeletal muscle.
慢性腎疾患における早期血管老化における反復体細胞変異とプロジェリン発現 Recurrent somatic mutation and progerin expression in early vascular aging of chronic kidney disease
Gwladys Revêchon,Anna Witasp,Nikenza Viceconte,Hafdis T. Helgadottir,Piotr Machtel,Fabiana Stefani,Daniel Whisenant,Agustin Sola-Carvajal,Dagmara McGuinness,Nadia O. Abutaleb,Gonzalo Artiach,Emelie Wallén Arzt,Inga Soveri,Anne Babler,Susanne Ziegler,Rafael Kramann,Magnus Bäck,Anders Thorell,George A. Truskey,Lars Wennberg,Paul G. Shiels,Annika Wernerson,Peter Stenvinkel & Maria Eriksson
Nature Aging Published:10 June 2025
DOI:https://doi.org/10.1038/s43587-025-00882-6
Abstract
Early vascular aging plays a central role in chronic kidney disease (CKD), but its molecular causes remain unclear. Somatic mutations accumulate in various cells with age, yet their functional contribution to aging tissues is not well understood. Here we found progerin, the protein responsible for the premature aging disease Hutchinson–Gilford progeria syndrome, steadily recurring in vascular smooth muscle cells of patients with CKD. Notably, the most common progeria-causing mutation, LMNA c.1824C>T, was identified as a somatic mutation in CKD arteries. Clusters of proliferative progerin-expressing cells in CKD arteries and in vivo lineage-tracing in mice revealed clonal expansion capacity of mutant cells. Mosaic progerin expression contributed to genomic damage, endoplasmic reticulum stress and senescence in CKD arteries and resulted in vascular aging phenotypes in vivo. These findings suggest that certain somatic mutations may be clonally expanded in the arterial wall, contributing to the disease-related functional decline of the tissue.