2025-08-22 順天堂大学,山梨大学,科学技術振興機構
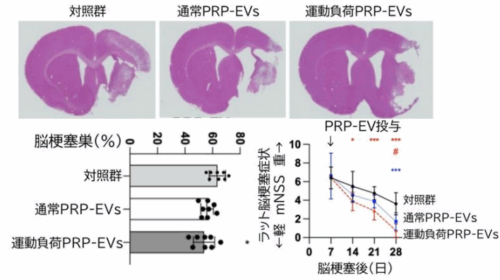
図1:脳梗塞作成後 28 日における脳梗塞巣・脳梗塞症状比較脳梗塞作成 7 日後(脳梗塞亜急性期から慢性期)に多血小板血漿から作成したエクソソーム(PRP-EVs)もしくは、運動負荷を行ったラットから抽出した PRP-EVs を投与し、比較したところ、対照群に比し、脳梗塞は縮小しており、mNSS(げっ歯類での脳梗塞症状評価項目)は軽減しました。
<関連情報>
- https://www.jst.go.jp/pr/announce/20250822-2/index.html
- https://www.jst.go.jp/pr/announce/20250822-2/pdf/20250822-2.pdf
- https://journals.sagepub.com/doi/10.1177/0271678X251369219
運動誘発性多血小板血漿由来の細胞外小胞は、虚血性脳卒中後の回復を改善した Exercise-induced extracellular vesicles derived from platelet-rich plasma improved recovery after ischemic stroke
Yoshifumi Miyauchi, Nobukazu Miyamoto, […], and Yuji Ueno
Journal of Cerebral Blood Flow & Metabolism Published:August 20, 2025
DOI:https://doi.org/10.1177/0271678X251369219
Abstract
Stroke remains a major global health burden, with limited treatments for chronic ischemic stroke necessitating novel therapies. This study explored the therapeutic potential of platelet-rich plasma (PRP)-derived extracellular vesicles (EVs) in stroke recovery, particularly in exercise-trained rats. PRP-derived EVs from treadmill-loaded and sedentary rats were designated athletes (aPRP-EVs) and non-athlete (nPRP-EVs), respectively. Both were administered to primary cortical neurons exposed to oxygen-glucose deprivation (OGD) and to adult male Wistar/ST rats subjected to permanent middle cerebral artery occlusion (MCAO). Exercise increased CD63, CD31, and transforming growth factor-β1 (TGF-β1) in PRP-derived EVs. In OGD-exposed neurons, aPRP-EVs enhanced viability, elevated phosphorylated neurofilament heavy chain, and reduced intracellular calcium. Canonical pathway analysis showed upregulated TGF-β/SMAD signaling in EV groups versus vehicle, while ‘Ca signaling’ was downregulated in aPRP-EVs versus nPRP-EVs. In MCAO rats, EVs improved neurological and motor function and reduced neuronal apoptosis at 28 days, with aPRP-EVs promoting earlier, greater recovery and infarct reduction. These effects correlated with TGF-β1 upregulation, SMAD4 nuclear translocation, reduced NMDAR2B expression, and enhanced axonal growth in the peri-infarct region. PRP-derived EVs, particularly from exercise-trained donors, enhance neuroregeneration and functional recovery in chronic ischemic stroke via TGF-β/SMAD and calcium signaling modulation.
