2025-08-26 バーミンガム大学
<関連情報>
- https://www.birmingham.ac.uk/news/2025/adding-cell-based-vaccine-to-liver-cancer-therapy-slows-cancer-progression-in-patients
- https://aacrjournals.org/clincancerres/article/31/16/3412/763982/Addition-of-Dendritic-Cell-Vaccination-to
肝細胞癌患者におけるシクロホスファミド前処置と化学塞栓療法への樹状細胞ワクチン追加:ImmunoTACE試験 Addition of Dendritic Cell Vaccination to Conditioning Cyclophosphamide and Chemoembolization in Patients with Hepatocellular Carcinoma: The ImmunoTACE Trial
Yuk Ting Ma;Jianmin Zuo;Amanda Kirkham;Stuart Curbishley;Miroslava Blahova;Anna L. Rowe;Camilla Bathurst;Homoyoon Mehrzad;Salil Karkhanis;Pankaj Punia;Martin W. James;Nick Stern;Ankit Rao;Diana Hull;Faye Lowe;Panagiota Sylla;Luke Webster;Syed Hussain;Christina Yap;Daniel Palmer;David H. Adams
Clinical Cancer Research Published:August 14 2025
DOI:https://doi.org/10.1158/1078-0432.CCR-25-0142
Abstract
Purpose:
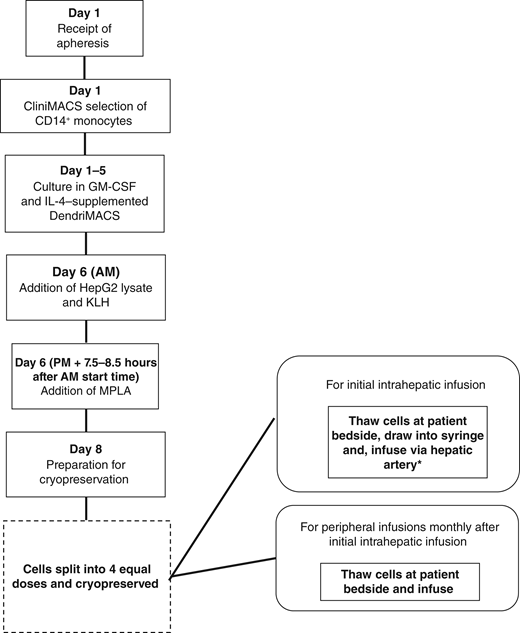
A previous study by our group using dendritic cells (DC) pulsed ex vivo with the lysate of the HepG2 cell line showed evidence of antigen-specific T-cell responses in some patients with advanced hepatocellular carcinoma. The ImmunoTACE trial evaluated the preliminary activity of this vaccine in combination with transarterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma.
Patients and Methods:
A randomized phase II trial was conducted in three tertiary referral centers in the United Kingdom. Eligible patients were randomly assigned in a 1:1 ratio to TACE + preconditioning cyclophosphamide or to TACE + preconditioning cyclophosphamide + DC infusions. The primary endpoint was progression-free survival time using RECIST v1.1 criteria. Additional endpoints included safety and immune responses.
Results:
Between March 2016 and October 2019, 55 patients were randomized, of whom 48 were evaluable (24 in each group). The median progression-free survival time using RECIST criteria was 18.6 months in patients treated with chemoembolization + preconditioning cyclophosphamide + DC infusions compared with 10.4 months in those treated with chemoembolization + preconditioning cyclophosphamide alone (HR = 0.43; upper value of one-sided 80% confidence interval, 0.57; P = 0.016). The addition of DC infusions did not significantly increase the incidence or severity of adverse events. An enhanced antigen (α-fetoprotein)-specific immune response was observed in patients treated with DC vaccination.
Conclusions:
The addition of DC infusions to TACE and preconditioning cyclophosphamide has shown promising preliminary activity and merits further investigation in a larger randomized trial.