2025-05-12 ワシントン大学セントルイス校
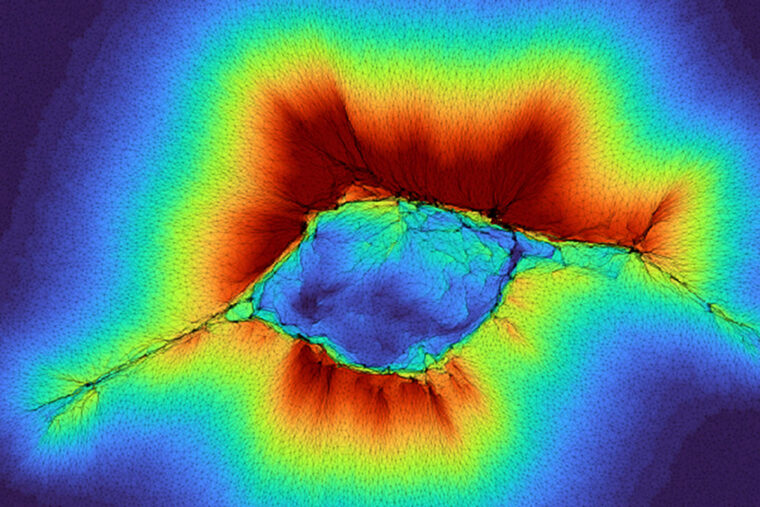
In this model, cell clusters can “feel” farther beyond their surrounding environment in the human body by acting as a collective, shown here “reaching out” with the red indicating the spatial distribution of the collagen deformation over a layer of stiff tissue. WashU researchers share details on this emergent property in the journal PNAS. (Image: Pathak lab)
<関連情報>
- https://source.washu.edu/2025/09/working-together-cells-extend-their-senses/
- https://engineering.washu.edu/news/2025/Working-together-cells-extend-their-senses.html
- https://www.pnas.org/doi/10.1073/pnas.2423875122
上皮集団の深部機械感覚の出現は層状マトリックス上の細胞のクラスター形成と分散を制御する Emergent depth-mechanosensing of epithelial collectives regulates cell clustering and dispersal on layered matrices
Hongsheng Yu and Amit Pathak
Proceedings of the National Academy of Sciences Published:September 11, 2025
DOI:https://doi.org/10.1073/pnas.2423875122
Significance
Previous work has shown that single cells can sense microenvironment stiffness through thin matrix layers, and this range extends with viscoelastic collagen compared to elastic hydrogels. Here, we found that epithelial cell collectives can mechanosense matrix stiffness over 100 μm deep into collagen layers. Stiffer basal matrix enables lower cell dispersal and higher clustering, enabled by collective collagen deformation and stiffening. According to our experiments and simulations, the inhibition of cellular contractility or intercellular adhesions disrupts this emergent phenomenon and disables depth mechanosensing. These findings expand the known length scales of conventional mechanosensing and suggest that cell clusters at tissue interfaces—such as in tumor invasion, wound healing, or organogenesis—mechanosense not only their adhered surfaces but also distant matrix layers.
Abstract
During wound healing, tumor growth, and organ formation, epithelial cells migrate and cluster in layered tissue environments. Although cellular mechanosensing of adhered extracellular matrices is now well recognized, it is unclear how deeply cells sense through distant matrix layers. Since single cells can mechanosense stiff basal surfaces through soft hydrogels of <10 μm thickness, here we ask whether cellular collectives can perform such “depth-mechanosensing” through thicker matrix layers. Using a collagen-polyacrylamide double-layer hydrogel, we found that epithelial cell collectives can mechanosense basal substrates at a depth of >100 μm, assessed by cell clustering and collagen deformation. On collagen layers with stiffer basal substrates, cells initially migrate slower while performing higher collagen deformation and stiffening, resulting in reduced dispersal of epithelial clusters. These processes occur in two broad phases: cellular clustering and dynamic collagen deformation, followed by cell migration and dispersal. Using a cell-populated collagen-polyacrylamide computational model, we show that stiffer basal substrates enable higher collagen deformation, which in turn extends the clustering phase of epithelial cells and reduces their dispersal. Disruption of collective collagen deformation, by either α-catenin depletion or myosin-II inhibition, disables the depth-mechanosensitive differences in epithelial responses between soft and stiff basal substrates. These findings suggest that depth-mechanosensing is an emergent property that arises from collective collagen deformation caused by epithelial cell clusters. This work broadens the conventional understanding of epithelial mechanosensing from immediate surfaces to underlying basal matrices, providing insights relevant to tissue contexts with layers of varying stiffness, such as wound healing and tumor invasion.