2025-10-07 国立精神・神経医療研究センター
<関連情報>
- https://www.ncnp.go.jp/topics/detail.php?@uid=wt6PHDLPxkDeP70I
- https://www.nature.com/articles/s43587-025-00980-5
アルツハイマー病抗アミロイド療法の安全な使用を最適化するための日本における患者レジストリ
A Japanese registry for optimizing the safe use of anti-amyloid therapies for Alzheimer’s disease in Japan
Takeshi Iwatsubo
Nature Aging Published:26 September 2025
DOI:https://doi.org/10.1038/s43587-025-00980-5
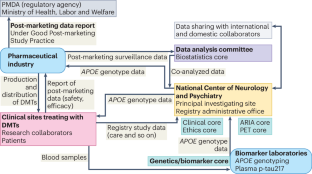
The Japanese regulatory authority has approved the clinical use of anti-amyloid-β (Aβ) immunotherapy for Alzheimer’s disease. This Correspondence highlights the launch of the Japanese Registry for Alzheimer’s Disease-Modifying Therapies, which aims to ensure safety and maximize the optimal risk–benefit balance through integrating a real-world database of adverse events, post-marketing surveillance, APOE genotyping and biomarker research.
Lecanemab, an anti-Aβ-antibody drug for Alzheimer’s disease, received accelerated approval in the USA on 6 January 2023 and was submitted for approval in Japan on 16 January. On 6 July, the US Food and Drug Administration granted full approval for lecanemab. Later that year, the Pharmaceuticals and Medical Devices Agency (PMDA) — the Japanese regulatory authority — approved the clinical use of lecanemab, which was listed in the national health insurance and made available for clinical use on 20 December 2023.

