2025-10-22 産業技術総合研究所
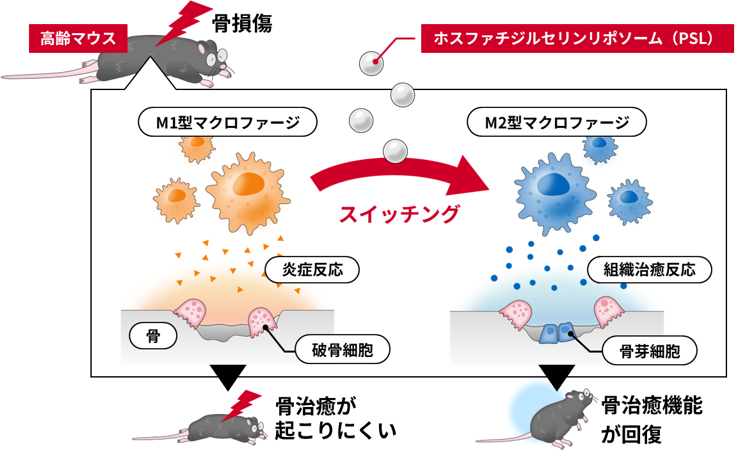
リポソームによる老化骨の治癒促進
<関連情報>
- https://www.aist.go.jp/aist_j/press_release/pr2025/pr20251022_2/pr20251022_2.html
- https://pubs.acs.org/doi/10.1021/acsami.5c15449
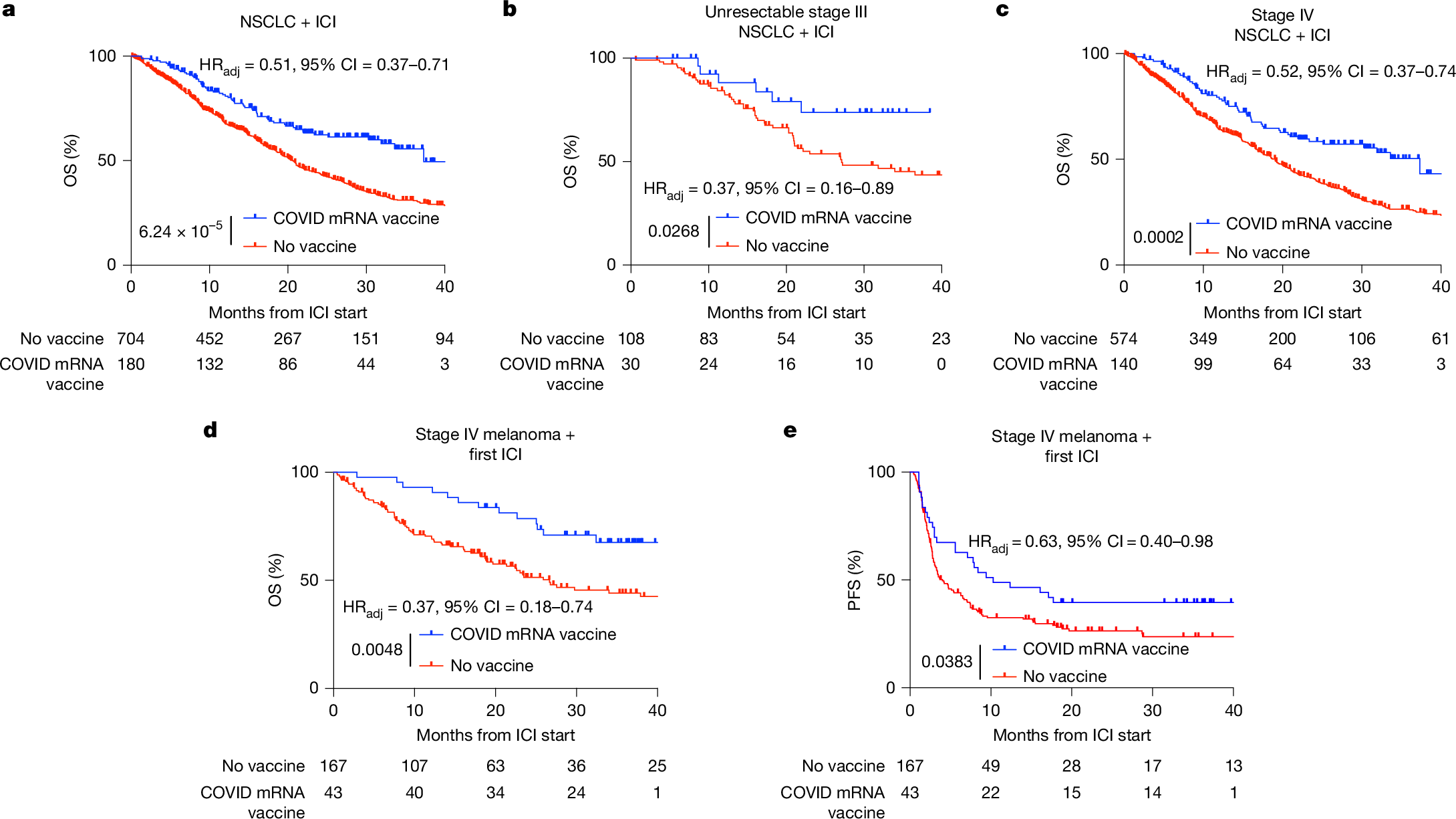
年齢と性別に依存しないマクロファージの極性化による老化骨損傷の再生促進 Accelerated Regeneration of Senescent Bone Injury through Age- and Sex-Independent Macrophage Polarization
Naoyuki Fukuda,Natsumi Takamaru,Jeong-Hun Kang,and Riki Toita
ACS Applied Materials & Interfaces Published October 15, 2025
DOI:https://doi.org/10.1021/acsami.5c15449
Abstract
Nanomedicines offer broad therapeutic potential, but key host factors such as age and sex (now recognized as critical factors for efficacy) remain largely overlooked. Aging, which is characterized by systemic chronic inflammation and delayed tissue regeneration, poses significant medical and economic issues in aging societies. Older individuals exhibit impaired macrophage transition from an inflammatory M1 phenotype to an anti-inflammatory/pro-healing M2 phenotype, and this transition is a potential target for rejuvenating tissue repair. Existing therapeutic approaches, such as cytokines and biomaterial surface engineering, effectively promote M1-to-M2 polarization in young individuals, but their efficacy is markedly reduced in older individuals, and sex differences in therapeutic macrophage polarization remain largely unexplored. Here, we show that phosphatidylserine liposomes (PSLs) induced macrophage polarization independent of age (3–4 months old or >21 months old) and sex in mice. In addition, in vitro experiments confirmed that factors secreted by M1 macrophages inhibited osteoblast (OB) differentiation and enhanced osteoclast (OC) differentiation, with older macrophages from both sexes exerting more pronounced effects, while factors secreted by M2 macrophages had the opposite effect. Furthermore, in a critical-sized bone defect model in old mice, PSLs induced macrophage phenotype conversion, improved the balance between OB and OC differentiation, and eventually accelerated bone repair. These findings suggest that PSLs are a universal M2 macrophage inducer and offer a promising therapeutic strategy for restoring bone repair in older individuals as well as potentially promoting tissue regeneration in other organs.