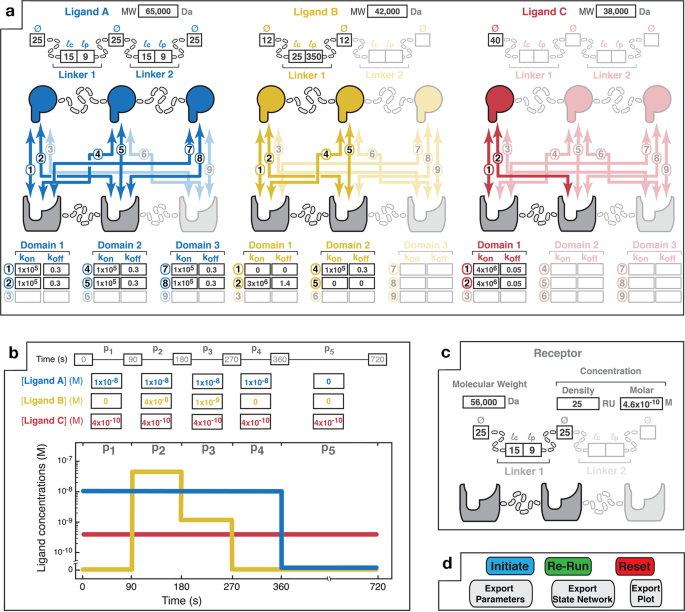
高リスク患者を特定することで、がん医療を改善できる可能性がある Identifying high-risk patients could improve cancer care
2022-09-01
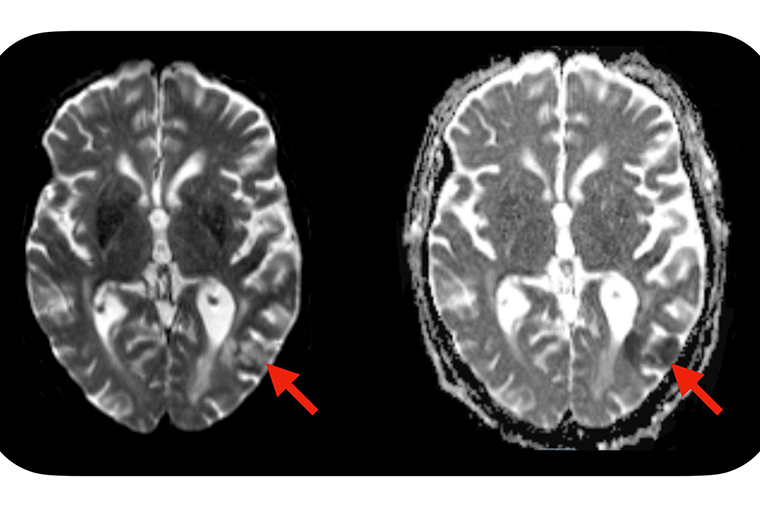
A new study from Washington University School of Medicine in St. Louis suggests a simple blood test — administered before CAR-T cell treatment is initiated — may identify which patients are predisposed to developing neurotoxic side effects after CAR-T cell therapy. Severe side effects can include seizures, brain swelling and strokes. Evidence of a stroke (red arrows) is seen on this MRI scan of the brain of a patient who developed neurotoxic side effects after CAR-T cell therapy. (Image: Omar Butt/School of Medicine)
<関連情報>
- https://source.wustl.edu/2022/09/simple-blood-test-predicts-neurotoxic-complications-of-car-t-cell-therapy/
- https://jamanetwork.com/journals/jamaoncology/fullarticle/2795985
免疫不全細胞関連神経毒性症候群を発症した患者における治療前および治療後のニューロフィラメント軽鎖レベルの推移の評価 Assessment of Pretreatment and Posttreatment Evolution of Neurofilament Light Chain Levels in Patients Who Develop Immune Effector Cell–Associated Neurotoxicity Syndrome
Omar H. Butt, Alice Y. Zhou, Paolo F. Caimi, Patrick H. Luckett, Julie K. Wisch, Paul-Robert Derenoncourt, Kenneth Lee, Gregory F. Wu, Marcos J. G. de Lima, Jian L. Campian, Matthew J. Frank, John F. DiPersio, Armin Ghobadi, Beau M. Ances
JAMA Oncology Published:September 1, 2022
DOI:doi:10.1001/jamaoncol.2022.3738
Key Points
Question How early are elevations in plasma neurofilament light chain (NfL) levels observed before the development of neurotoxicity after chimeric antigen receptor T-cell therapy?
Findings In this cross-sectional study of 30 patients undergoing cellular therapy, NfL levels were elevated in patients who developed immune effector cell–associated neurotoxicity syndrome (ICANS) at baseline (preinfusion) and remained elevated for 30 days.
Meaning Preinfusion NfL elevations suggest ICANS may unmask preexisting neurologic injury present prior to infusion, suggesting that early screening is feasible before drug administration and that inclusion of preexisting neurologic injury may be considered in ICANS pathophysiology models traditionally focusing on the interaction of inflammation and endothelial dysfunction.
Abstract
Importance Determining whether neurofilament light chain (NfL) elevations in patients who develop immune effector cell–associated neurotoxicity syndrome (ICANS) occur before or after infusion of cellular product is important to identify high-risk patients and inform whether neuroaxonal injury is latent or a consequence of treatment.
Objective To quantify serial NfL levels in patients undergoing cellular therapy.
Design, Setting, and Participants This retrospective 2-center study examined plasma NfL levels in 30 patients with detailed medical and treatment history, including all major pretreatment and posttreatment risk factors. Exclusion criteria included dementia and severe, symptomatic central nervous system (CNS) involvement.
Main Outcomes and Measures Patients’ NfL levels were measured at 7 time points: baseline (prelymphodepletion), during lymphodepletion, postinfusion day (D) 1, D3, D7, D14, and D30. Prediction accuracy for the development of ICANS was next modeled using receiver operating characteristic (ROC) classification. Finally, univariate and multivariate modeling examined the association between NfL levels, ICANS, and potential risk factors including demographic (age, sex), oncologic (tumor burden, history of CNS involvement), neurologic (history of nononcologic CNS disease or neuropathy), and neurotoxic exposure histories (vincristine, cytarabine, methotrexate, or CNS radiotherapy).
Results A total of 30 patients (median [range] age, 64 [22-80] years; 12 women [40%] and 18 men [60%]) were included. Individuals who developed ICANS had elevations in NfL prior to lymphodepletion and chimeric antigen receptor T-cell infusion compared with those who did not develop ICANS (no ICANS: 29.4 pg/mL, vs any ICANS: 87.6 pg/mL; P < .001). Baseline NfL levels further predicted ICANS development with high accuracy (area under the ROC curve, 0.96), sensitivity (0.91), and specificity (0.95). Levels of NfL remained elevated across all time points, up to 30 days postinfusion. Baseline NfL levels correlated with ICANS severity but not demographic factors, oncologic history, nononcologic neurologic history, or history of exposure to neurotoxic therapies.
Conclusions and Relevance In a subset of patients in this cross-sectional study, the risk of developing ICANS was associated with preexisting neuroaxonal injury that was quantifiable with plasma NfL level. This latent neuroaxonal injury was present prior to drug administration but was not associated with historic neurotoxic therapies or nononcologic neurologic disease. Preinfusion NfL may further permit early screening and identification of patients most at risk for ICANS. Additional studies are needed to determine NfL’s utility as a predictive biomarker for early (preemptive or prophylactic) intervention and to delineate the origin of this underlying neural injury.