2025-10-24 理化学研究所,東京大学,早稲田大学
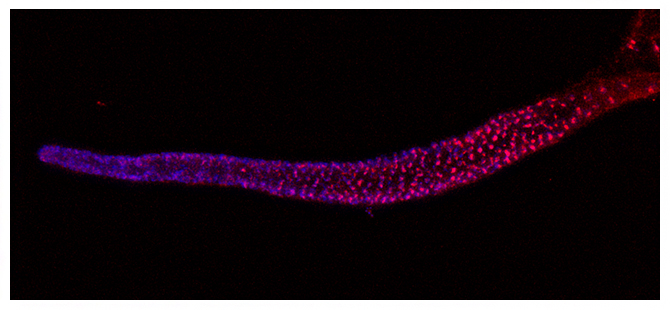
イントロン・ヒッチハイクにより線虫の生殖細胞(青)で発現するテロメレースRNA(赤)
<関連情報>
- https://www.riken.jp/press/2025/20251024_1/index.html
- https://www.science.org/doi/10.1126/science.ads7778
線虫テロメラーゼRNAは生殖細胞系列で発現が上昇する遺伝子のイントロンにヒッチハイクする Nematode telomerase RNA hitchhikes on introns of germline–up-regulated genes
Yutaka Takeda, Masahiro Onoguchi, Fumiya Ito, Io Yamamoto, […] , and Hiroki Shibuya
Science Published:23 Oct 2025
DOI:https://doi.org/10.1126/science.ads7778
Editor’s summary
To ensure species survival, organisms must transmit fully elongated telomeric DNA to the next generation. Without complete elongation, telomeric DNA would progressively shorten with each generation, ultimately leading to DNA loss and lineage extinction. Telomerase, a ribonucleoprotein complex, facilitates telomere elongation, but its tissue-specific activation mechanism has remained elusive. Takeda et al. showed that in Caenorhabditis elegans, a newly identified telomerase RNA subunit, terc-1, hitchhikes within the introns of the protein-coding gene nmy-2. Transplanting terc-1 into the introns of germline-expressed genes ensured germline immortality, suggesting that nematode telomerase RNA exploits introns of germline–up-regulated genes to secure species survival. —Di Jiang
Structured Abstract
INTRODUCTION
Telomeric DNA at chromosome ends shortens with each cell division, ultimately triggering cellular senescence. Telomerase, a reverse transcriptase composed of a catalytic subunit and an RNA template, becomes active in certain cell types, such as cancer cells and stem cells, to counteract this shortening and enable sustained cell proliferation. Germ cells also exhibit high telomerase activity, not only to support germline stem cell proliferation but also to transmit sufficiently long telomeres to offspring, thereby safeguarding species survival. Indeed, germ cells maintain longer telomeres than most somatic progenitor cells throughout the organism’s lifespan. However, the mechanisms underlying tissue-specific telomerase activation and transgenerational inheritance of telomeric DNA remain poorly understood.
RATIONALE
To study transgenerational telomere maintenance, we took advantage of the short generation time (~3 days) of the nematode Caenorhabditis elegans. Despite being a widely used model organism, telomerase RNA has not been identified in nematodes, possibly because it is expressed from an atypical genomic locus that eludes standard gene annotation, unlike canonical telomerase RNA genes transcribed from their own promoters.
RESULTS
To search for the long-elusive nematode telomerase RNA, we generated a C. elegans strain overexpressing FLAG-TRT-1, the catalytic subunit of telomerase. Biochemical purification of FLAG-TRT-1–associated RNAs using enhanced cross-linking and immunoprecipitation (eCLIP) identified an intronic long noncoding RNA (lncRNA), termed terc-1 (telomerase RNA component 1), within intron 2 of nmy-2, a germline-up-regulated gene encoding nonmuscle myosin II. Mutations in terc-1 caused progressive telomere shortening and sterility over generations, establishing terc-1 as the first functional telomerase RNA expressed as an intronic lncRNA.
Putative terc-1 orthologs were found within nmy-2 introns in C. briggsae and C. japonica, demonstrating that the intronic origin of terc-1 might be evolutionarily conserved in nematodes. Despite overall sequence divergence, in vitro and in vivo RNA structure probing revealed conserved features, including the CR4/5 domain, H/ACA box, and a nematode-specific stem, but no clear evidence for the canonical pseudoknot structure in their 5′ region.
An independent eCLIP screen targeting the dyskerin complex, essential for H/ACA box small nucleolar RNA (snoRNA) biogenesis, identified terc-1 as a dyskerin-bound RNA. Its expression depends on dyskerin and its cofactor, TCAB-1. Furthermore, the terc-1 precursor, spliced from nmy-2 pre-mRNA, is polyadenylated and then trimmed by the nuclear RNA exosome to generate the mature terc-1, indicating a maturation pathway distinct from that of other metazoan telomerase RNAs.
To investigate whether the genomic location is crucial for terc-1 function, we transplanted its sequence into introns of tissue-specific genes. Only insertions into germline-expressed genes restored germline immortality and rescued population survival. Furthermore, expression of terc-1 exclusively in the germline lineage was sufficient to maintain telomeres across generations, indicating that telomere length in C. elegans is primarily determined by the germline.
CONCLUSION
Our findings uncovered a species survival strategy in which telomerase RNA extends telomeres for the next generation by hitchhiking on introns of germline-expressed genes. This unexpected regulatory mechanism, termed “intron hitchhiking,” underscores the evolutionary adaptability of telomerase RNA and may reflect a broader strategy used by other noncoding RNAs involved in germline development and heritable trait transmission.

Intron hitchhiking by the C. elegans telomerase RNA.
A previously unidentified telomerase RNA (terc-1) in C. elegans is discovered in this study. terc-1 is expressed as an intronic lncRNA and undergoes splicing-dependent maturation. By hitchhiking on the introns of germline-expressed genes, terc-1 becomes active in germ cells, thereby ensuring transgenerational telomere maintenance and species survival. [Diagram created with BioRender.com]
Abstract
Telomerase is a ribonucleoprotein complex that elongates telomeric DNA, ensuring germline immortality. In this study, we identified the Caenorhabditis elegans telomerase RNA component 1 (terc-1), as the first known telomerase RNA expressed as an intronic long noncoding RNA (lncRNA), embedded in an intron of germline–up-regulated gene nmy-2. terc-1 undergoes splicing, polyadenylation, and nuclear RNA exosome–dependent maturation, stabilized by H/ACA small nucleolar ribonucleoproteins, thus co-opting the H/ACA small nucleolar RNA (snoRNA) biogenesis machinery. Mutations in terc-1 led to progressive telomere shortening and sterility in successive generations. Artificially transplanting the nmy-2 intron into the introns of germline-expressed genes but not non–germline-expressed genes restored germline immortality, highlighting the importance of genomic context. Our findings suggest that nematode telomerase RNA is a snoRNA-like intronic lncRNA that exploits the introns of germline–up-regulated genes to ensure species survival.