2025-11-03 マウントサイナイ医療システム(MSHS)
Web要約 の発言:
<関連情報>
- https://www.mountsinai.org/about/newsroom/2025/a-specific-human-gene-can-help-the-heart-repair-itself-from-heart-attack-or-heart-failure
- https://www.nature.com/articles/s41536-025-00438-7
- https://www.science.org/doi/abs/10.1126/scitranslmed.3007668
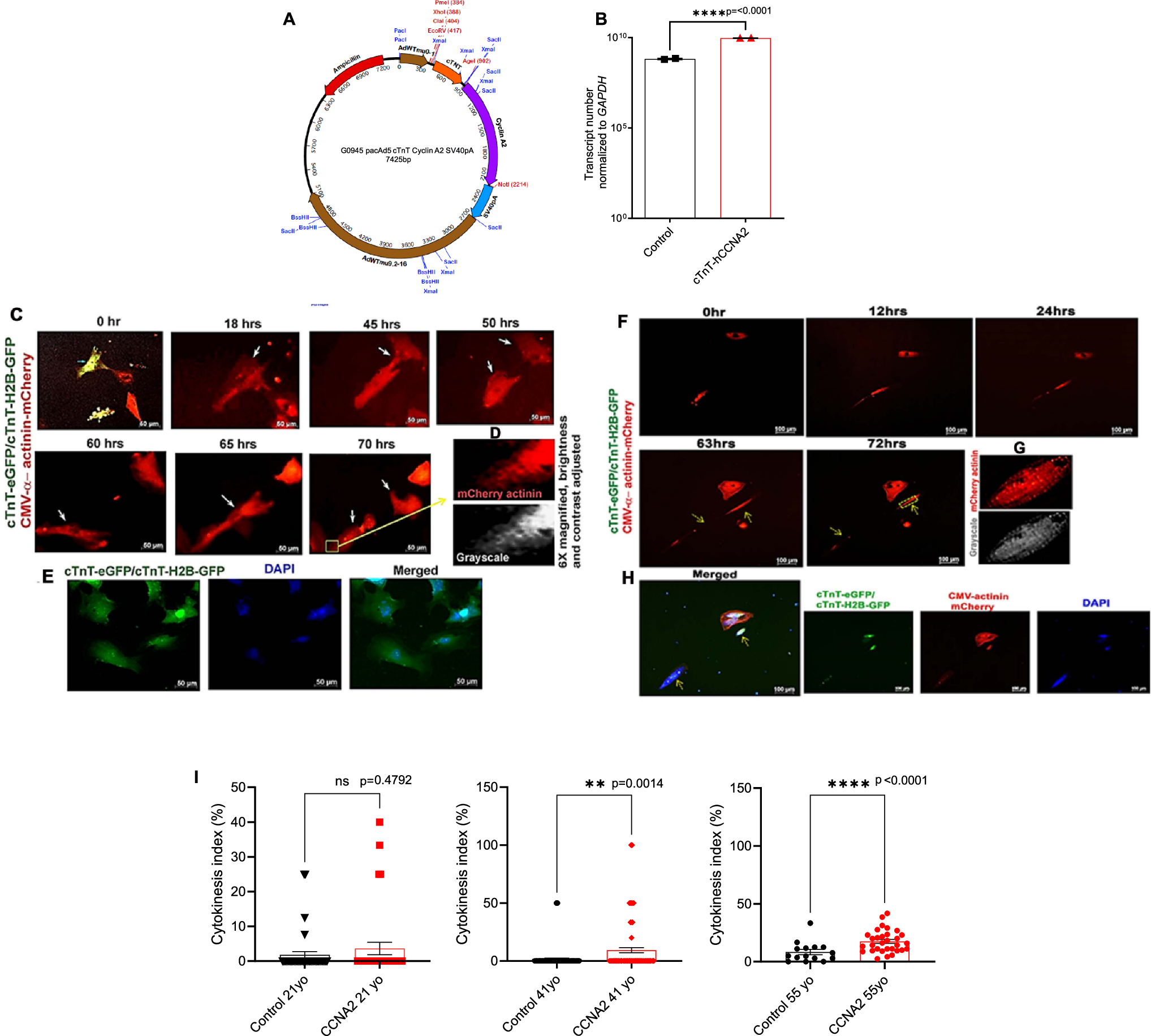
サイクリンA2はヒト成人心筋細胞の細胞質分裂を誘導し、マウスの再プログラミングを促進する Cyclin A2 induces cytokinesis in human adult cardiomyocytes and drives reprogramming in mice
Esmaa Bouhamida,Sangeetha Vadakke-Madathil,Prabhu Mathiyalagan,Amaresh K. Ranjan,Amir Khan,Cherrie D. Sherman,Paul E. Miller,Andre Ghetti,Najah Abi-Gerges & Hina W. Chaudhry
npj Regenerative Medicine Published:03 November 2025
DOI:https://doi.org/10.1038/s41536-025-00438-7
Abstract
Cyclin A2 (CCNA2), a master cell cycle regulator silenced in postnatal cardiomyocytes, promotes cardiac repair in animal models. However, its effect on cytokinesis in adult human cardiomyocytes was previously unknown. We engineered a replication-deficient adenoviral vector encoding human CCNA2 under the cardiac Troponin T promoter and delivered it to freshly isolated cardiomyocytes from adult human hearts. Time-lapse live imaging revealed the induction of complete cytokinesis with preservation of sarcomeres and calcium mobilization in redifferentiated daughter cardiomyocytes. Single-nucleus transcriptomic profiling of CCNA2-transgenic and non-transgenic mouse hearts uncovered a cardiomyocyte subpopulation characterized by enrichment of cytokinesis, proliferation, and reprogramming gene signatures. Ultra-deep bulk RNA sequencing of adult and fetal human hearts further highlighted reprogramming pathways relevant to CCNA2-induced effects. Together, these findings demonstrate that CCNA2 can reinitiate cytokinesis in adult human cardiomyocytes, illuminating conserved molecular programs that support its promise as a regenerative gene therapy for the heart.
サイクリンA2は成人心筋細胞の細胞分裂を介して心筋梗塞後の心臓再生を誘導する Cyclin A2 Induces Cardiac Regeneration After Myocardial Infarction Through Cytokinesis of Adult Cardiomyocytes
Scott D. Shapiro, Amaresh K. Ranjan, Yoshiaki Kawase, Richard K. Cheng, […] , and Hina W. Chaudhry
Science Translational Medicine Published:19 Feb 2014
DOI:https://doi.org/10.1126/scitranslmed.3007668
A Change of Heart After Myocardial Infarction
When blood flow is blocked off to the heart, the heart suffers permanent damage in part because cardiomyocytes are terminally differentiated and cannot proliferate. But what if these cells could be stimulated to divide? Some animals—like newts—have the ability to regenerate body parts when they are injured. Others—like zebrafish—can even regenerate heart tissue. Now, Shapiro et al. report that gene therapy can elicit a regenerative response in pig hearts.
Cyclin A2 (Ccna2) has been shown to induce cardiac repair in small-animal models after myocardial infarction (MI). The authors have extended these studies by looking in the more translationally relevant pig model of MI. They found that Ccna2 delivered by an adenovirus improved heart function when compared with an adenoviral control. Cardiomyocytes in the pigs showed evidence of increased proliferation. If these data hold true in human studies, patients with MI can take heart.
Abstract
Cyclin A2 (Ccna2), normally silenced after birth in the mammalian heart, can induce cardiac repair in small-animal models of myocardial infarction. We report that delivery of the Ccna2 gene to infarcted porcine hearts invokes a regenerative response. We used a catheter-based approach to occlude the left anterior descending artery in swine, which resulted in substantial myocardial infarction. A week later, we performed left lateral thoracotomy and injected adenovirus carrying complementary DNA encoding CCNA2 or null adenovirus into peri-infarct myocardium. Six weeks after treatment, we assessed cardiac contractile function using multimodality imaging including magnetic resonance imaging, which demonstrated ~18% increase in ejection fraction of Ccna2-treated pigs and ~4% decrease in control pigs. Histologic studies demonstrate in vivo evidence of increased cardiomyocyte mitoses, increased cardiomyocyte number, and decreased fibrosis in the experimental pigs. Using time-lapse microscopic imaging of cultured adult porcine cardiomyocytes, we also show that Ccna2 elicits cytokinesis of adult porcine cardiomyocytes with preservation of sarcomeric structure. These data provide a compelling framework for the design and development of cardiac regenerative therapies based on cardiomyocyte cell cycle regulation.