2025-11-05 北海道大学
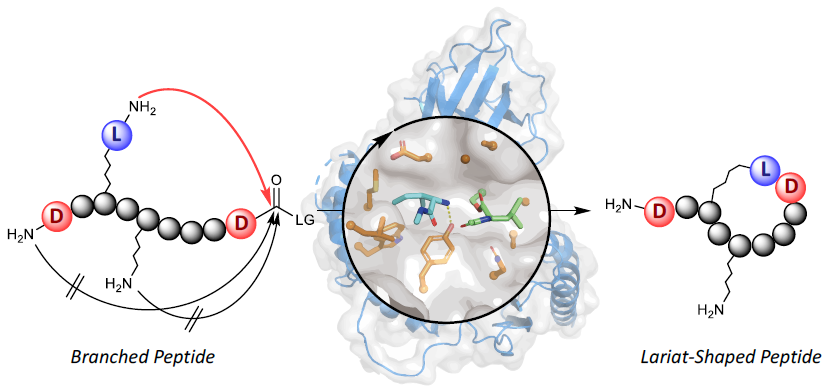
非リボソームペプチド環化酵素によるラリアット環の選択的な構築
<関連情報>
- https://www.hokudai.ac.jp/news/2025/11/–10.html
- https://www.hokudai.ac.jp/news/pdf/251105_pr.pdf
- https://www.nature.com/articles/s41557-025-01979-6
非リボソームペプチドによるラリアットリポペプチドの化学-酵素合成 Non-ribosomal peptide cyclase-directed chemoenzymatic synthesis of lariat lipopeptides
Masakazu Kobayashi,Kenichi Matsuda,Yuito Yamada,Rintaro Ichihara,Naho Onozawa,Hanako Fukano,Yoshihiko Hoshino,Aki Hirabayashi,Masato Suzuki,Akira Katsuyama,Satoshi Ichikawa & Toshiyuki Wakimoto
Nature Chemistry Published:04 November 2025
DOI:https://doi.org/10.1038/s41557-025-01979-6
Abstract
Lariat-shaped lipopeptides are important antimicrobial agents; however, their complex structures pose synthetic challenges that hamper efficient structural diversification. Here we report a new chemoenzymatic approach that facilitates access to lariat-shaped macrocycles. Unprotected, branched peptides bearing multiple nucleophiles, including a native amino terminus and a pseudo-amino terminus, were site-selectively cyclized using versatile non-ribosomal peptide cyclases, generating an array of lariat peptides with diverse sequences and ring sizes. The generality of this strategy was demonstrated using two penicillin-binding protein-type thioesterases, SurE and WolJ, as well as one type-I thioesterase, TycC thioesterase. Furthermore, the remaining nucleophile, which was not involved in the cyclization process, was exploited as a reactive handle for subsequent diversification via a site-selective acylation reaction (that is, Ser/Thr ligation). The tandem cyclization–acylation strategy enabled the one-pot, modular synthesis of lariat-shaped lipopeptides equipped with various acyl groups. Biological screening revealed that the site-selective acylation endowed the macrocyclic scaffolds with antimycobacterial activity and led to the identification of lipopeptides that inhibit 50% of growth at concentrations of 8–16 µg ml-1.