2025-11-06 東京科学大学
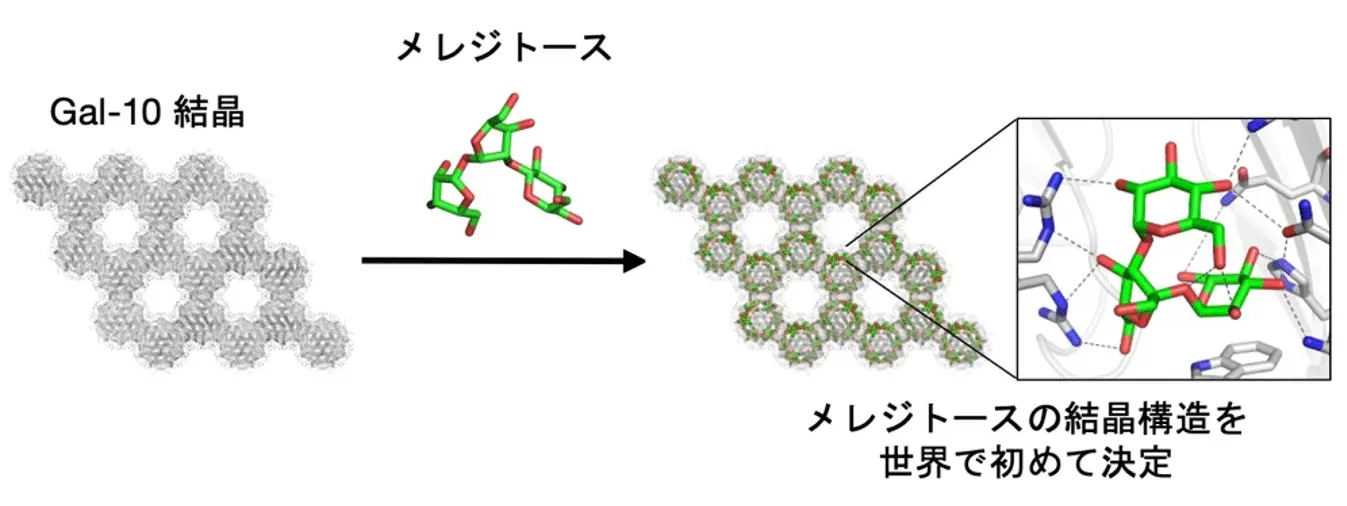
図1.Gal-10結晶に捕捉された三糖類「メレジトース」の構造解析
<関連情報>
- https://www.isct.ac.jp/ja/news/g3ptzrblym6q
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2605&prevId=&key=9784c15dfea25a159e34895112067ee7.pd
- https://onlinelibrary.wiley.com/doi/10.1002/sstr.202500501
無細胞タンパク質結晶化により、ガレクチン-10結晶を用いた二糖類および三糖類の迅速な構造決定が可能に Cell-Free Protein Crystallization Enables Rapid Structure Determination of Disaccharides and Trisaccharides Using Galectin-10 Crystals
Mariko Kojima, Xinchen Yao, Satoshi Abe, Tadaomi Furuta, Kunio Hirata, Ririko Kobayashi, Taiga Suzuki, Takafumi Ueno
Small Structures Published: 23 October 2025
DOI:https://doi.org/10.1002/sstr.202500501
Abstract
It is critical to understand the conformational selection and dynamics of flexible saccharides via protein–ligand interactions in efforts to elucidate their biofunctional roles. Protein crystals can serve as scaffolds to immobilize small molecules, enabling structural and dynamic analysis of saccharides that are difficult to study by conventional approaches. However, constructing versatile scaffold crystals for high-throughput structural analysis remains challenging because this work involves laborious protein production and crystallization workflows. Herein, rapid crystallization and structural analysis of saccharide-bound scaffolds are enabled by applying cell-free protein crystallization (CFPC) to galectin-10 (Gal-10), a lectin known to crystallize spontaneously in vivo. Using CFPC-generated Gal-10 crystals, the first atomic-resolution structure of melezitose, one of the trisaccharides, bound to the protein scaffold is obtained, revealing binding modes inaccessible by conventional approaches. Normalized B-factor analysis combined with molecular dynamics simulations reveals how the binding-site architecture modulates saccharide flexibility and immobilization. This platform can be extended to other flexible ligands and fragment-based screening.

