2025-11-13 東京科学大学
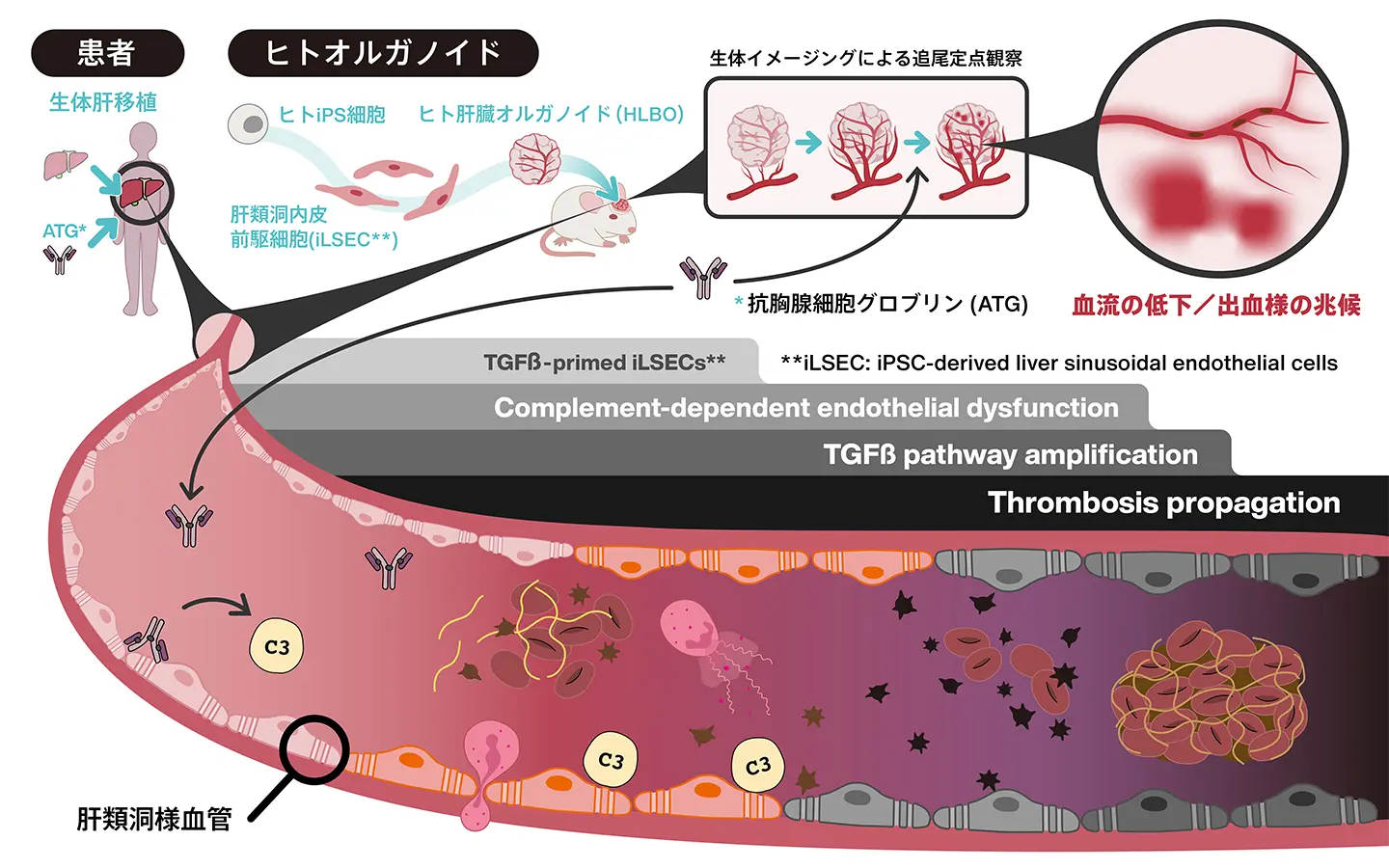
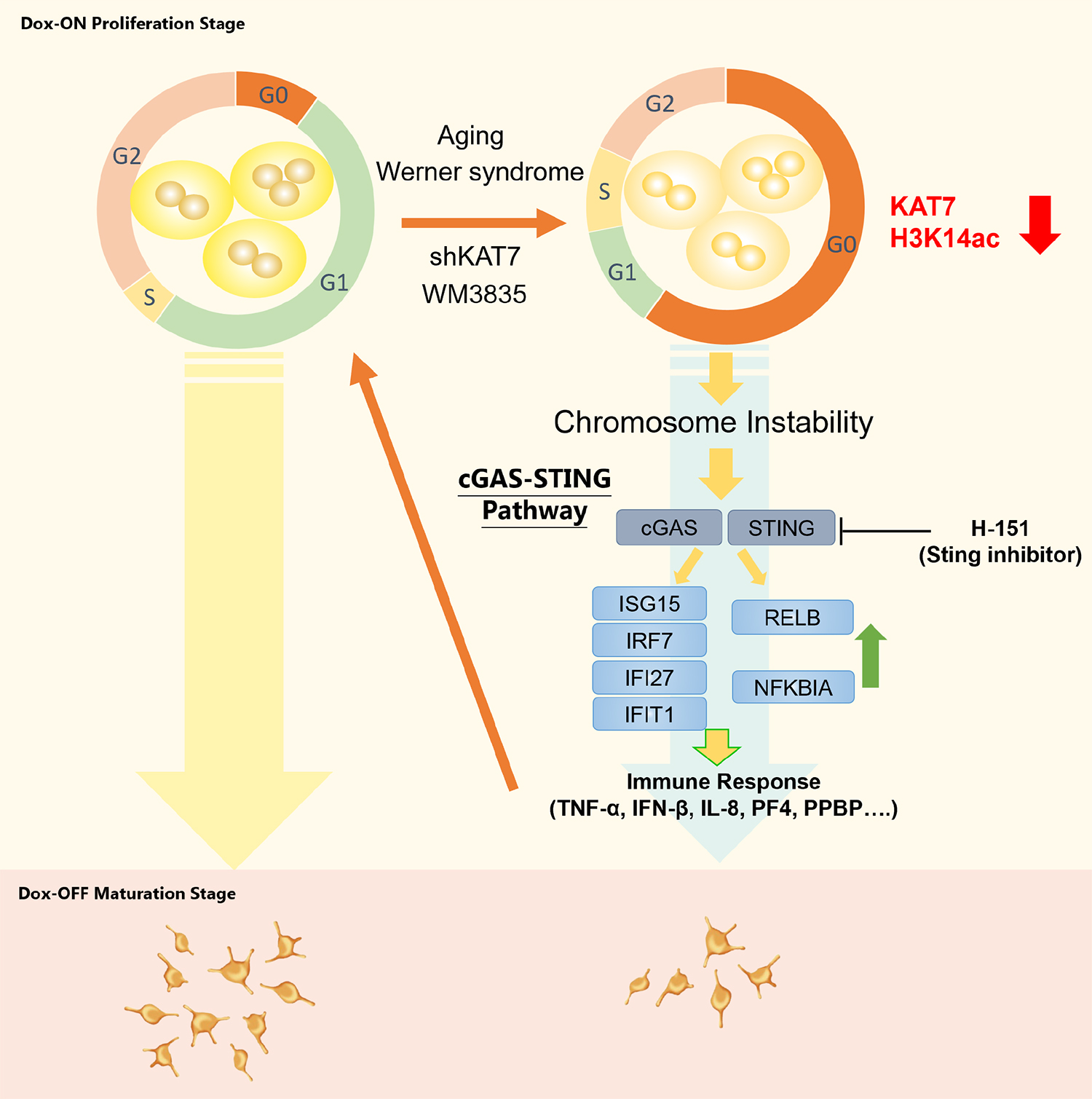
図1. 研究成果の概要
ヒト類洞構造を有する肝臓オルガノイドに、臓器移植後の免疫抑制剤であるATGを投与し、段階的に血栓形成が進行するメカニズムを解明した。
<関連情報>
- https://www.isct.ac.jp/ja/news/8svcawudjqjd
- https://www.isct.ac.jp/plugins/cms/component_download_file.php?type=2&pageId=&contentsId=1&contentsDataId=2648&prevId=&key=581bdecff827c2861d0e8604b922ebe0.pdf
- https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00506-3
ヒトiPSC由来血管新生肝オルガノイドを用いた抗胸腺細胞グロブリン誘発性微小血管症のモデル化 Modeling antithymocyte globulin-induced microvasculopathy using human iPSC-derived vascularized liver organoids
Shuntaro Kawamura ∙ Yosuke Yoneyama, ∙ Norikazu Saiki, ∙ … ∙ Yuta Hirata ∙ Yasunaru Sakuma ∙ Takanori Takebe
Cell Reports Medicine Published:November 6, 2025
DOI:https://doi.org/10.1016/j.xcrm.2025.102433
Highlights
- iPSC-derived vascularized liver organoids model ATG-induced hepatic microvasculopathy
- ATG triggers complement-dependent early thrombosis in human liver sinusoids
- ATG induces delayed proinflammatory responses of human LSECs with neutrophil degranulation
- TGF-β pathway activation in human LSECs exacerbates ATG-driven prothrombotic state
Summary
Antithymocyte globulin (ATG) is a widely used immunosuppressive agent, yet its off-target vascular effects remain a clinical challenge in part due to a lack of relevant human models. Here, we uncover a biphasic mechanism of ATG-induced microvasculopathy using a humanized liver organoid platform derived from induced pluripotent stem cells (iPSCs). We show that ATG triggers a rapid, complement-dependent phase of injury restricted to human iPSC-derived liver sinusoidal endothelial cells (iLSECs), leading to C3 deposition and acute thrombosis specifically in human but not in mouse vessels. Transcriptomics reveals a delayed transforming growth factor β (TGF-β) pathway-driven proinflammatory program that coincides with neutrophil recruitment and degranulation, while pharmacological TGF-β blockade attenuates thrombosis and flow disturbance. Our findings reveal a pathogenic sequence of complement and TGF-β pathway activation and establish a translational platform for dissecting human liver-specific microvasculopathy in vivo.