2025-12-03 ピッツバーグ大学
<関連情報>
- https://news.engineering.pitt.edu/renerva-inc-approved-for-first-in-human-fda-clinical-trials/
- https://www.nature.com/articles/s41536-025-00416-z
神経キャップ移植デバイスによる神経成長と誘発性疼痛の予防 Prevention of nerve growth and evoked pain with a nerve cap graft device
Sydney Borcherding,Matthew D. Wood,Sai L. Pinni,Lauren Schellhardt,Anne E. Faust,Marissa N. Behun,Clint Skillen,Pooja Chawla,Mangesh Kulkarni,Elena A. Demeter,Andrew D. Miller,Mark A. Mahan,Bryan N. Brown &Lorenzo Soletti
npj Regenerative Medicine Published:07 June 2025
DOI:https://doi.org/10.1038/s41536-025-00416-z
Abstract
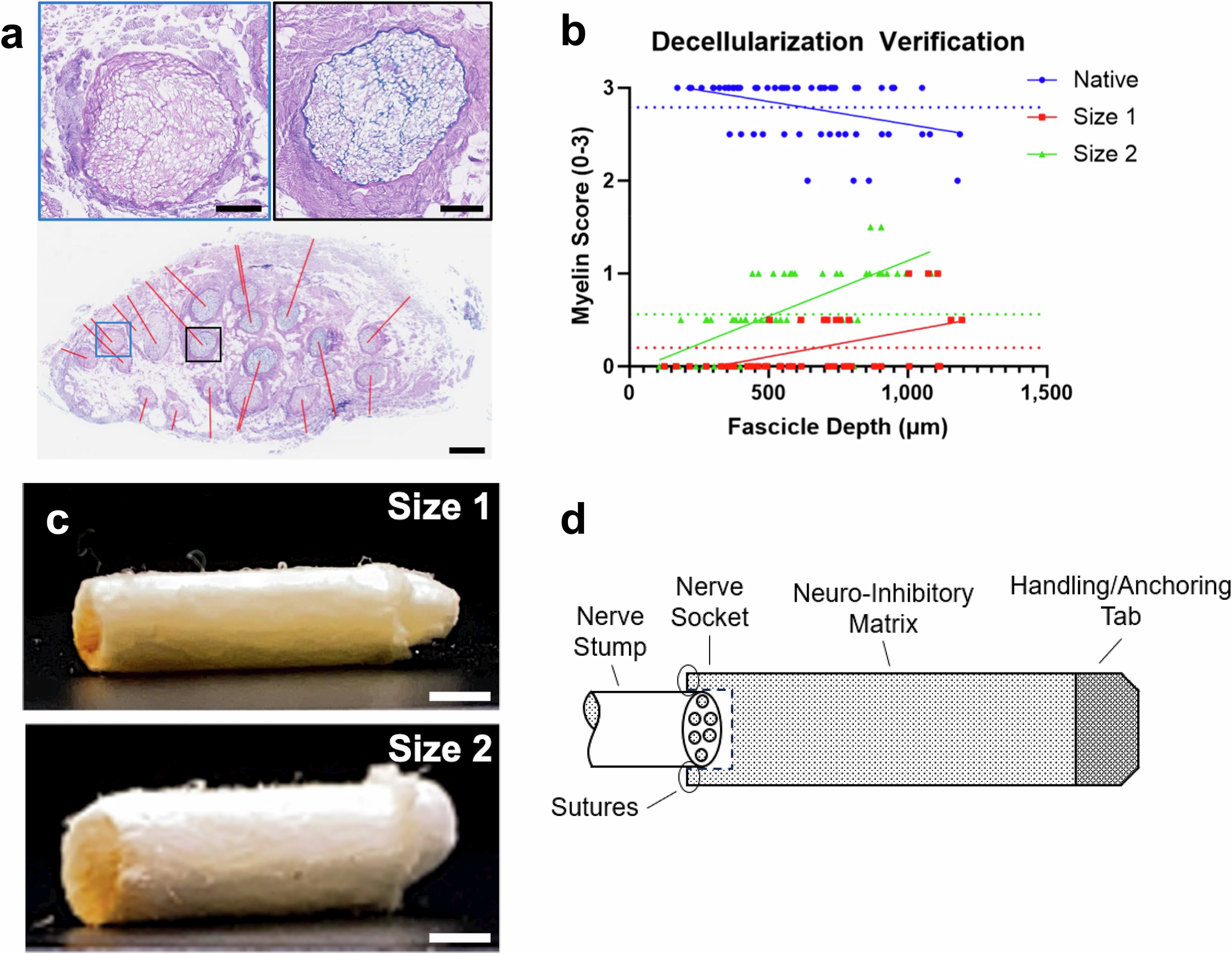
Neuroma following nerve injury and/or amputation is a debilitating condition with significant impacts on quality of life. Several approaches exist to prevent or treat neuroma and/or reduce associated pain; however, these approaches are not consistently effective, facile, or widely accessible. The present study characterizes a xenogeneic nerve cap graft device (NCGD) composed of decellularized porcine nerve. The NCGD was assessed for its ability to inhibit nerve growth, neuroma formation, and pain in rodent models of sciatic neurectomy and tibial neuroma transposition. The NCGD provided a neuroinhibitory substrate that abated and interrupted nerve growth within 5 mm of the nerve stump and was progressively remodeled into healthy host-derived tissue. The NCGD also resulted in a 3.5-fold reduction in evoked pain and a decrease in pain-associated markers at the dorsal root ganglia. These results suggest that the NCGD may provide a simple and widely accessible alternative for prophylactic treatment of symptomatic neuroma.

