2025-12-08 カロリンスカ研究所(KI)
<関連情報>
- https://news.ki.se/lower-doses-of-immunotherapy-for-skin-cancer-give-better-results
- https://academic.oup.com/jnci/advance-article/doi/10.1093/jnci/djaf327/8372006
進行切除不能悪性黒色腫患者における反転投与量NIVO3+IPI1の評価 Evaluation of the flipped dose NIVO3+IPI1 in patients with advanced unresectable melanoma
Karl Björkström, MD, PhD ,Cissi Liu, MD ,Anna Fager, MD ,Lisa L Liu, MD, PhD ,Lars Ny, MD, PhD ,Hildur Helgadottir, MD, PhD
Journal of the National Cancer Institute Published:08 December 2025
DOI:https://doi.org/10.1093/jnci/djaf327
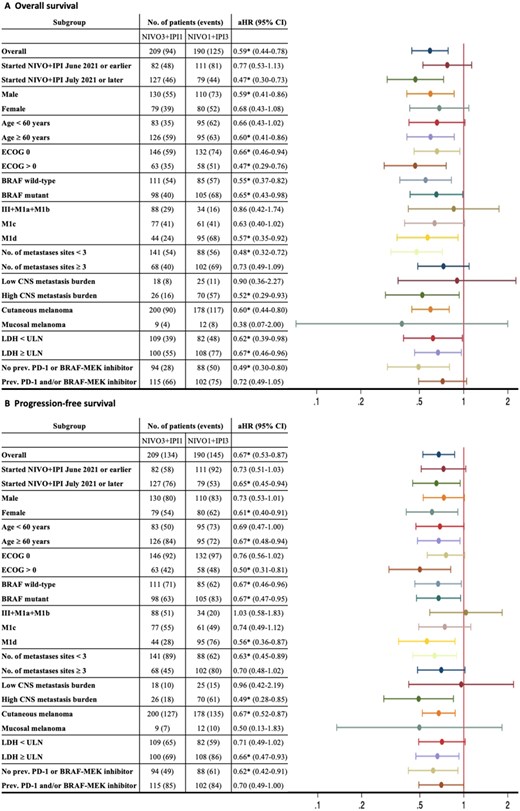
Abstract
Nivolumab 1 mg/kg plus ipilimumab 3 mg/kg (NIVO1+IPI3) was approved for advanced melanoma in 2016. The CheckMate 511 trial demonstrated improved tolerability with the flipped dose, NIVO3+IPI1, but this regimen has not been approved in melanoma by regulatory authorities. In this study, patients with advanced unresectable melanoma treated with NIVO3+IPI1 or NIVO1+IPI3 were included. The objective response rate was 48.8% with NIVO3+IPI1 (n = 209) and 36.9% with NIVO1+IPI3 (n = 190) (P = .016). Adjusted hazard ratio (aHR) was 0.67 (95% CI = 0.53 to 0.87, P = .002) for progression-free survival and 0.59 (95% CI = 0.44 to 0.78, P < .001) for overall survival (OS). In most studied subgroups aHR was <1, in favor of NIVO3+IPI1. The incidence of grade 3-5 immune-related adverse events was 30.6% with NIVO3+IPI1 vs. 51.1% with NIVO1+IPI3 (P < .001). This study shows that in a real-world setting, NIVO3+IPI1 demonstrated superior efficacy compared with NIVO1+IPI3, possibly related to a beneficial safety and tolerability profile allowing for more received doses.