2025-12-10 アメリカ国立衛生研究所 (NIH)
<関連情報>
- https://www.nih.gov/news-events/news-releases/nih-led-study-reveals-role-mobile-dna-elements-lung-cancer-progression
- https://www.nature.com/articles/s41586-025-09825-y
肺腺癌の進化におけるLINE-1の役割の解明 Uncovering the role of LINE-1 in the evolution of lung adenocarcinoma
Tongwu Zhang,Wei Zhao,Christopher Wirth,Marcos Díaz-Gay,Jinhu Yin,Monia Cecati,Francesca Marchegiani,Phuc H. Hoang,Charles Leduc,Marina K. Baine,William D. Travis,Lynette M. Sholl,Philippe Joubert,Jian Sang,John P. McElderry,Michelle Antony,Alyssa Klein,Azhar Khandekar,Caleb Hartman,Jennifer Rosenbaum,Frank J. Colón-Matos,Mona Miraftab,Monjoy Saha,Olivia W. Lee,… Maria Teresa Landi
Nature Published:10 December 2025
DOI:https://doi.org/10.1038/s41586-025-09825-y
Abstract
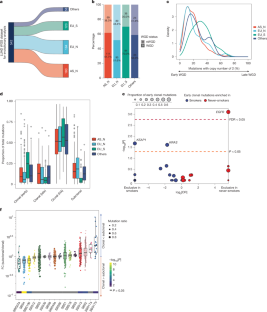
Understanding lung cancer evolution can identify tools for intercepting its growth1,2. Here, in a landscape analysis of 1,024 lung adenocarcinomas (LUADs) with deep whole-genome sequencing integrated with multiomic data, we identified 542 LUADs with a diverse clonal architecture. In this group, we observed divergent evolutionary trajectories based on tobacco smoking exposure, ancestry and sex. LUAD from smokers showed an abundance of tobacco-related C:G>A:T driver mutations3 in KRAS and short subclonal diversification. LUAD in people who have never smoked (hereafter, never-smokers) showed early occurrence of copy-number alterations and EGFR mutations associated with SBS5 and SBS40a mutational signatures. Tumours containing EGFR mutations exhibited long latency, particularly in female individuals of European-ancestry. Tumours from Asian never-smokers showed a short clonal evolution. Importantly, we found that the mutational signature ID24 is a marker of a previously unrecognized mechanism for LUAD evolution. Tumours with ID2 showed short latency and high long interspersed nuclear element-1 (LINE-1, hereafter L1) retrotransposon activity linked to L1 promoter demethylation. These tumours exhibited an aggressive phenotype with genomic instability, elevated hypoxia scores, low neoantigen burden, metastasis propensity and poor overall survival. Reactivated L1-retrotransposition-induced mutagenesis probably contributes to the mutational signature ID2, including through the regulation of the transcriptional factor ZNF695, a member of the KZFP family5. The complex nature of LUAD evolution creates both challenges and opportunities for screening and treatment plans.