2025-12-12 千葉大学
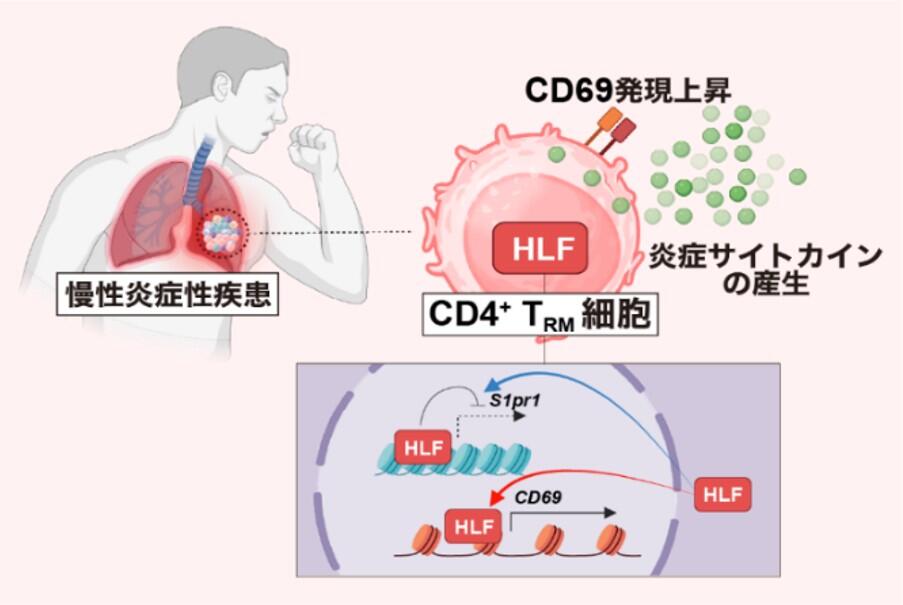
図:HLFはぜんそくを誘導する炎症性CD4+TRM細胞を制御する
<関連情報>
- https://www.chiba-u.ac.jp/news/research-collab/1212_cd4.html
- https://www.chiba-u.ac.jp/news/files/pdf/1212_cd4.pdf
- https://www.science.org/doi/10.1126/science.adp0714
肝白血病因子は炎症誘発性記憶CD4 + T細胞の組織内居住を誘導する Hepatic leukemia factor directs tissue residency of proinflammatory memory CD4+ T cells
Masahiro Kiuchi, Masahiro Nemoto, Hiroyuki Yagyu, Ami Aoki, […] , and Kiyoshi Hirahara
Science Published:11 Dec 2025
DOI:https://doi.org/10.1126/science.adp0714
Editor’s summary
Tissue-resident memory (TRM) cells provide defense against recurring infections, but their dysfunction can lead to disease. Although transcriptional programs associated with the formation of CD8+ TRM cells have been defined, much less is known about the development of CD4+ TRM cells. Kiuchi et al. identified a transcription factor, hepatic leukemia factor (HLF), that promoted the differentiation of CD4+ TRM cells in the lungs of mice during chronic inflammation. Based on its DNA-binding profile, HLF coordinated the upregulation of genes required for tissue residency and a proinflammatory phenotype while suppressing the expression of genes that are required for tissue egress. Mice that lacked HLF expression in CD4+ T cells had less lung pathology during long-term exposure to a fungal antigen. —Sarah H. Ross
Structured Abstract
INTRODUCTION
Tissue-resident memory T (TRM) cells residing in nonlymphoid barrier organs play crucial roles in host defense against pathogens and contribute to the pathogenesis of chronic inflammatory diseases. TRM cells are characterized by the up-regulation of tissue retention molecules such as CD69 and CD103 and the down-regulation of lymphoid homing molecules such as Ccr7 and S1pr1. Recent research has revealed distinct transcriptional programs underlying CD8+ TRM cell development, with factors such as Blimp1, Hobit, and Runx3 orchestrating their formation. However, molecules regulating CD4+ TRM cell development and functional heterogeneity remain poorly understood.
RATIONALE
Understanding the molecular mechanisms controlling CD4+ TRM cells is crucial for elucidating the pathogenesis of various chronic inflammatory diseases across multiple organ systems. Chronic airway inflammatory diseases represent a major global health burden, and severe cases are often resistant to conventional therapies, thus necessitating the identification of potential therapeutic targets. This study aimed to identify transcription factors controlling CD4+ TRM cell tissue residency and function using an Aspergillus fumigatus antigen–induced chronic airway inflammation model.
RESULTS
Single-cell RNA sequencing of murine lung tissue revealed that hepatic leukemia factor (Hlf), a member of the bZIP transcription factor family, showed the highest differential expression between tissue-resident versus circulating CD4+ T cells. In a mouse model of chronic airway inflammation, Hlf-deficient mice exhibited reduced numbers of lung CD4+ TRM cells and ameliorated airway inflammation, accompanied by decreased fibrotic responses. Chromatin immunoprecipitation sequencing analysis revealed that HLF directly bound to and repressed tissue egress genes, including S1pr1, Klf2, and Tcf7, while directly promoting the expression of tissue retention molecules, including CD69 and the transcription factor Bhlhe40. Hlf-deficient CD4+ TRM cells exhibited increased S1pr1 and decreased CD69 expression, indicating impaired tissue retention. Hlf-deficient TRM cells produced fewer inflammatory cytokines such as interleukin-5 (IL-5), IL-13, IL-17, and interferon-γ (IFN-γ). In patients with eosinophilic chronic rhinosinusitis, HLF+ CD4+ TRM cells in nasal polyps showed enhanced tissue residency signatures and inflammatory cytokine expression compared with HLF– cells.
CONCLUSION
This study identifies HLF as a transcriptional regulator of CD4+ TRM cell tissue residency and proinflammatory function, acting through coordinated enhancement of tissue retention programs and suppression of egress mechanisms. This regulatory circuit appeared to be conserved across species and multiple inflammatory diseases. HLF represents a potential driver of pathogenic CD4+ TRM cell subsets in chronic inflammation. These findings provide molecular insights into tissue-specific immunity and identify HLF as a potential therapeutic target for treating intractable chronic inflammatory diseases.

HLF regulates CD4+ TRM cell retention and inflammation in the lung.
HLF promotes the expression of CD69 and Bhlhe40 and suppresses the expression of S1pr1, thereby facilitating CD4+ TRM cell retention within inducible bronchus-associated lymphoid tissue (iBALT) and enhancing proinflammatory cytokine production in the lung. By contrast, HLF deficiency reduces CD69 and Bhlhe40, increases S1pr1, leads to enhanced egress of CD4+ T cells from the lung, and results in attenuated local inflammation. [Figure created with BioRender.com]
Abstract
CD4+ tissue-resident memory T (TRM) cells contribute to host defense and to the pathogenesis of chronic inflammatory diseases, but the molecules that direct their differentiation are unknown. We found that the transcription factor hepatic leukemia factor (HLF) could direct the tissue residency program and function of CD4+ TRM cells. HLF simultaneously up-regulated tissue retention receptors, down-regulated tissue egress receptors, and promoted proinflammatory CD4+ TRM cells by inducing Bhlhe40, and all of these processes were associated with changes in chromatin accessibility. Genetic deletion of Hlf inhibited CD4+ TRM cell generation and ameliorated airway tissue inflammation in vivo. HLF+ CD4+ TRM cells isolated from inflamed airway tissue in humans had a tissue residency signature and expressed inflammatory cytokines. We conclude that HLF may act as a central regulator of proinflammatory CD4+ TRM cell development and function.