2025-12-12 カリフォルニア大学リバーサイド校(UCR)
Zaaijer, S., Groen, S.C./Commun Med/2025
<関連情報>
- https://news.ucr.edu/articles/2025/12/12/fda-drug-trials-exclude-widening-slice-americans
- https://www.nature.com/articles/s43856-025-01270-2
米国FDA承認薬341品目における長期臨床試験登録動向と、その精密医療戦略における指導的役割 Longitudinal clinical trial enrollment trends across 341 US FDA-approved drugs and their guiding role in precision medicine strategies
Sophie Zaaijer & Simon C. Groen
Communications Medicine Published:05 December 2025
DOI:https://doi.org/10.1038/s43856-025-01270-2
Abstract
Background
Healthcare continues to suffer from a one-size-fits-all, trial-and-error model. Precision medicine seeks individualized care but requires clinical trial cohorts that mirror patient populations to detect differences in treatment response. Systematic assessments of trial cohort representativeness remain sparse; most studies are narrow. This study evaluates whether recent efforts, including the US FDA’s Drug Trial Snapshots Program, have improved demographic representation in pivotal trials across disease areas.
Methods
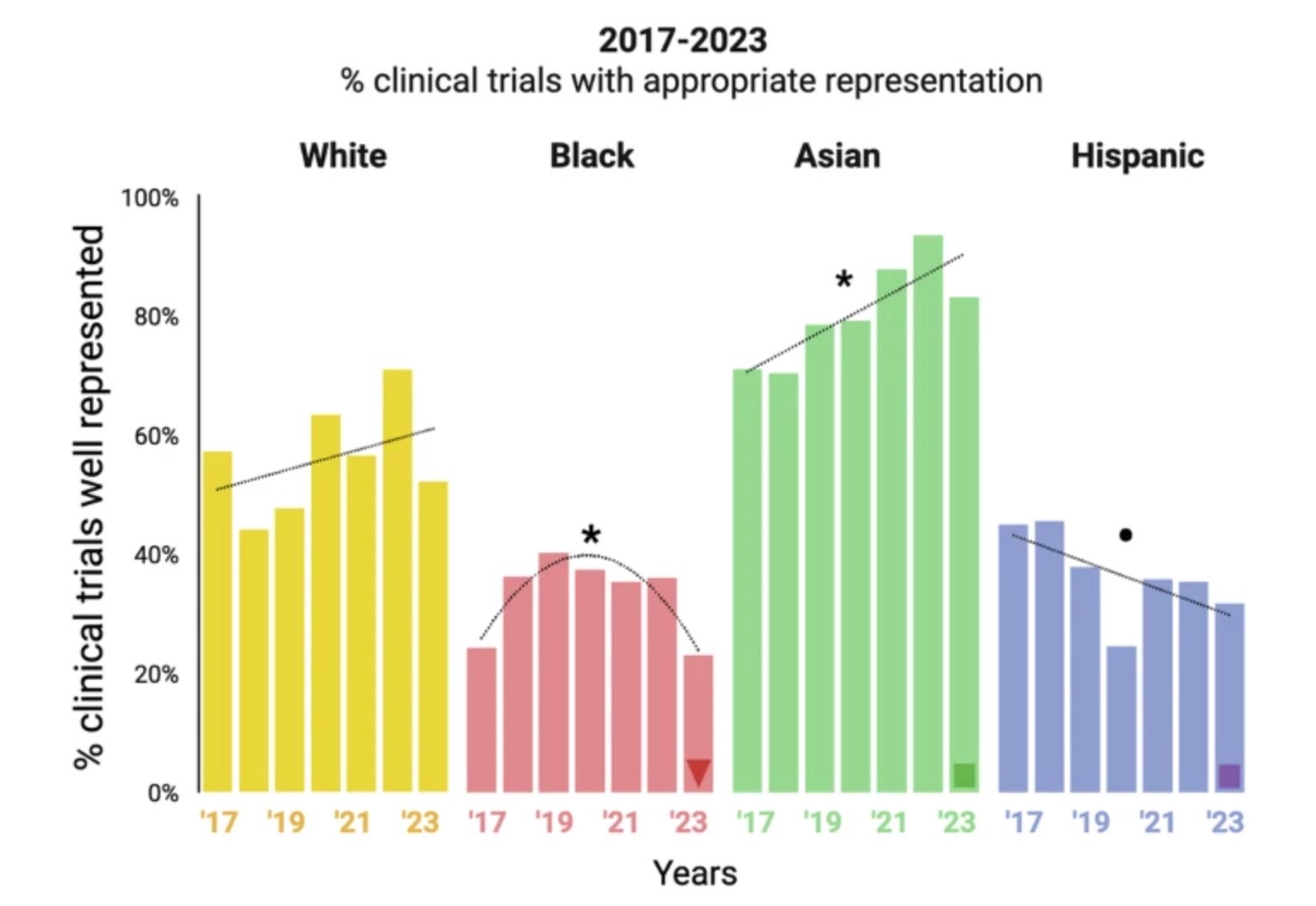
We analyzed Drug Trial Snapshots Program data covering 341 Phase III clinical trials supporting drug approvals between 2017 and 2023. We compared enrollment of Asian, Black, Hispanic, and White participants with US Census data using chi-squared tests; assessed trends with regression; and examined patterns by disease area, regulatory designation, and US recruitment.
Results
Here, we show that only 6% of pivotal clinical trials achieve enrollment aligned with the distribution of the four largest racial and ethnic groups in the US population. Enrollment of Black and Hispanic participants decreases over time, whereas that of Asian and White participants increases and remains stable, respectively. Trials based in the US include more Black participants, while trials with Breakthrough Therapy designation (which expedites development and review of drugs for serious or life-threatening conditions) show more balanced enrollment across groups.
Conclusions
Persistent imbalances in trial enrollment limit the delivery of precision medicine. Trial location and regulatory designations influence who is included. Earlier planning of trials and more strategic site selection improve the potential for future treatments to serve individuals from all racial and ethnic groups.
Plain language summary
Precision medicine tailors treatments to individuals. Therefore, trials for new therapies should enroll participants who reflect the populations that will use them. We analyzed enrollment in 341 trials supporting US Food and Drug Administration (FDA) approvals from 2017–2023. Black and Hispanic people were often underrepresented, while Asian and White people were better represented. This imbalance limits evaluation of genetic differences that may affect drug response. Improvements were seen in US-based trials and in those with Breakthrough Therapy designation, an expedited FDA pathway for serious conditions. We recommend practical steps: set representation targets early, use US-based sites when appropriate, and leverage regulatory tools. These measures support safer, more effective treatments for all communities and move health care toward precision medicine.


