2025-12-16 ペンシルベニア州立大学(Penn State)
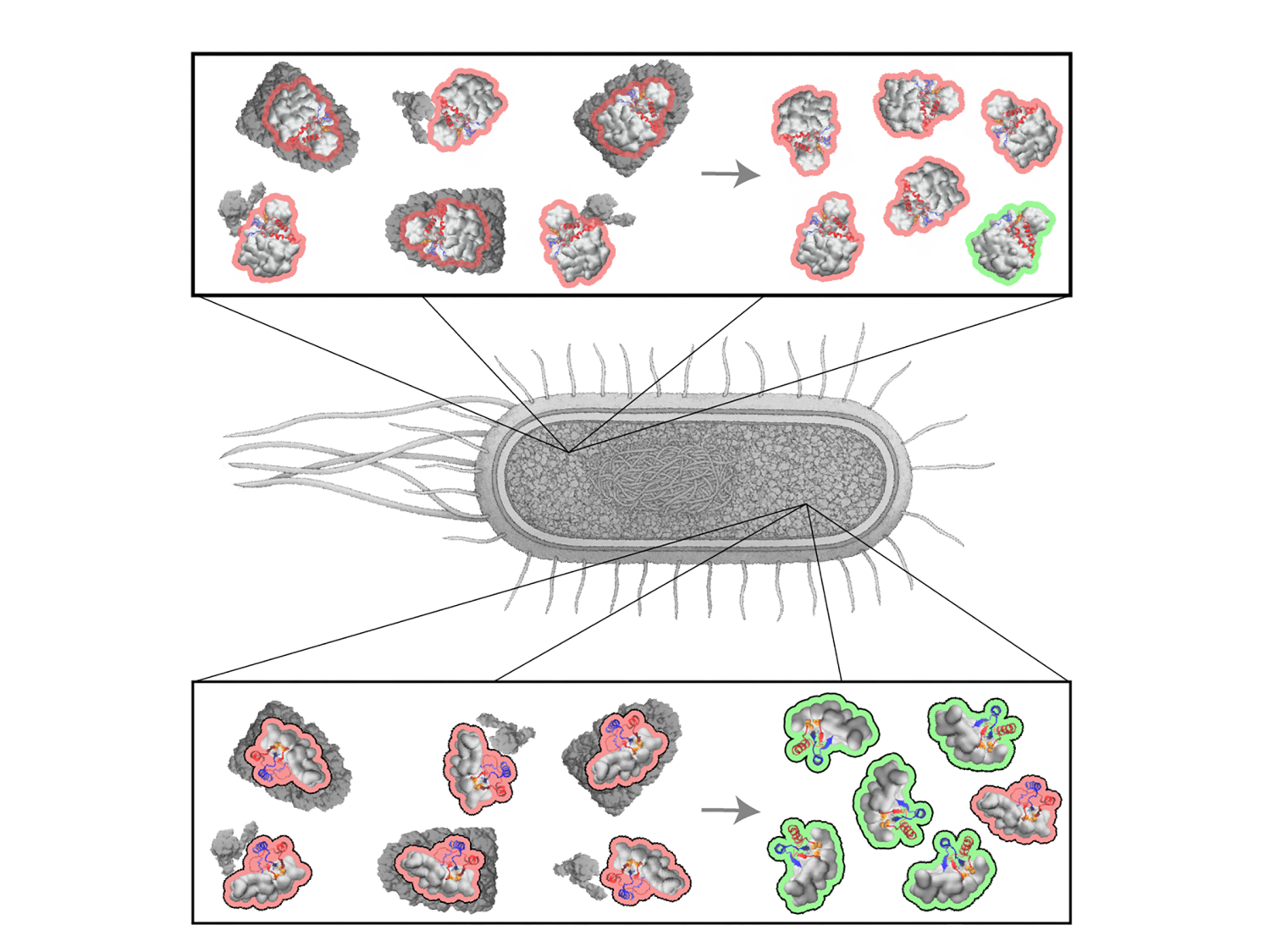
A new high-throughput study that used publicly available data shows that E. coli proteins containing a structure called a non-covalent lasso entanglement (NCLE) are more likely to misfold and, if they are essential to the bacteria’s survival, more likely to be repaired by chaperones — the cell’s quality control machinery. Image shows that misfolded (outlined in red) essential proteins (bottom) containing NCLEs are more likely to be repaired (outlined in green) by cellular chaperones (dark grey) than non-essential proteins (top). Credit: Ian Sitarik/O’Brien Lab / Penn State. Creative Commons
<関連情報>
- https://www.psu.edu/news/eberly-college-science/story/certain-life-essential-proteins-e-coli-repair-more-likely
- https://www.nature.com/articles/s41467-025-66236-3
広く見られるタンパク質ミスフォールディング機構は、遺伝子の必須性に基づいてシャペロンによってin vitroで差別的に救済される A widespread protein misfolding mechanism is differentially rescued in vitro by chaperones based on gene essentiality
Ian Sitarik,Quyen V. Vu,Justin Petucci,Paulina Frutos,Hyebin Song & Edward P. O’Brien
Nature Communications Published:12 December 2025
DOI:https://doi.org/10.1038/s41467-025-66236-3
Abstract
Protein misfolding involving changes in non-covalent lasso entanglement (NCLE) status has been proposed based on simulations and biochemical assays of a small number of proteins. Here, we detect hallmarks of these misfolded states across hundreds of proteins by integrating E. coli proteome-wide limited-proteolysis mass spectrometry data with structural datasets of protein native structures. Proteins containing native NCLEs are twice as likely to misfold, predominantly in regions where these NCLEs naturally occur. Surprisingly, the chaperones DnaK and GroEL do not typically correct this misfolding, except in the case of essential proteins. Statistical analysis links this differential rescue activity to weaker loop-closing contacts in the NCLEs of essential proteins, suggesting misfolding involving these loops is easier to rectify by chaperones. Molecular simulations indicate a mechanism where premature NCLE loop closure, prior to proper placement of the threading segment, leads to persistent misfolded states. This mechanism can explain why, in this mass spectrometry dataset, proteins with NCLEs are more likely to misfold and misfold in NCLE regions. These results suggest the potential for widespread NCLE misfolding, that such misfolded states in non-essential proteins could bypass the refolding action of chaperones, and that some protein sequences may have evolved to allow chaperone rescue from this class of misfolding.