2025-12-16 ロックフェラー大学
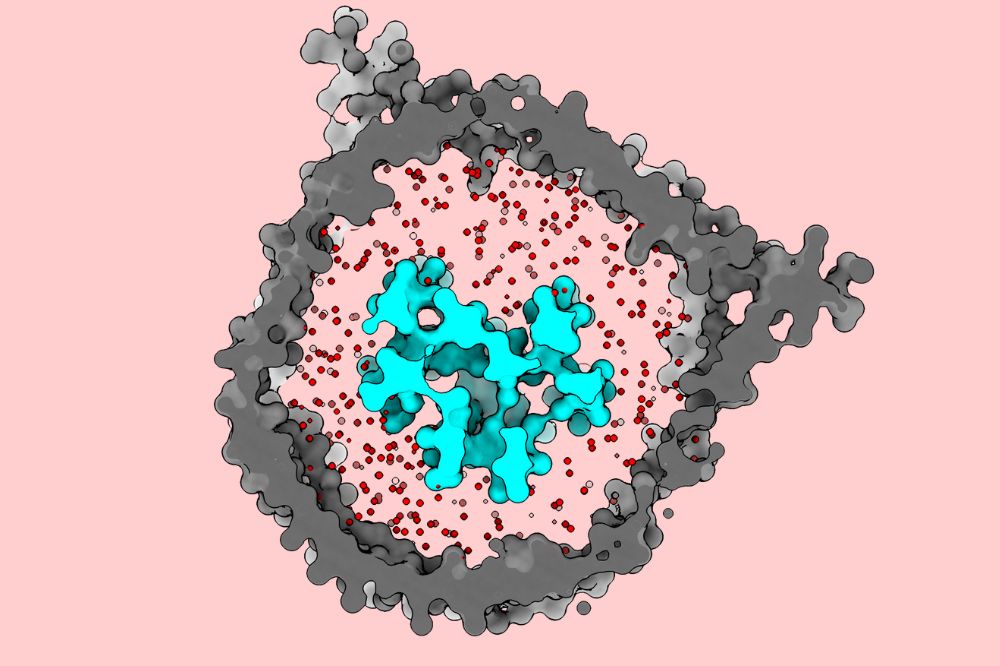 The gray portion of this schematic represents a nanodisc, where for the first time researchers were able to replicate the native membrane environment (red dots) of a T cell receptor (cyan). (Courtesy of the Walz lab)
The gray portion of this schematic represents a nanodisc, where for the first time researchers were able to replicate the native membrane environment (red dots) of a T cell receptor (cyan). (Courtesy of the Walz lab)
<関連情報>
- https://www.rockefeller.edu/news/38542-this-new-understanding-of-t-cell-receptors-may-improve-cancer-immunotherapies/
- https://www.nature.com/articles/s41467-025-66939-7
膜包埋ヒトT細胞受容体-CD3複合体の静止状態とリガンド結合状態 The resting and ligand-bound states of the membrane-embedded human T-cell receptor–CD3 complex
Ryan Q. Notti,Fei Yi,Søren Heissel,Martin W. Bush,Zaki Molvi,Pujita Das,Henrik Molina,Christopher A. Klebanoff & Thomas Walz
Nature Communications Published:16 December 2025
DOI:https://doi.org/10.1038/s41467-025-66939-7
Abstract
The T-cell receptor (TCR) initiates T-lymphocyte activation, but the mechanism of TCR activation remains uncertain. Here, we present cryogenic electron microscopy structures for the unliganded and human leukocyte antigen (HLA)-bound human TCR–CD3 complex in nanodiscs that provide a native-like lipid environment. Distinct from the open and extended conformation seen in detergent, the unliganded TCR–CD3 in nanodiscs adopts two related closed and compacted conformations that represent its physiologic resting state in vivo. By contrast, the HLA-bound complex adopts the open and extended conformation, and conformation-locking disulfide mutants show that ectodomain opening is necessary for maximal ligand-dependent T-cell activation. These structures also reveal conformation-dependent protein–lipid and glycan–glycan interactions within the TCR. Together, these results establish allosteric conformational change during TCR activation, reveal avenues for immunotherapeutic engineering, and highlight the importance of native-like lipid environments for membrane protein structure determination.