2025-12-25 東京大学
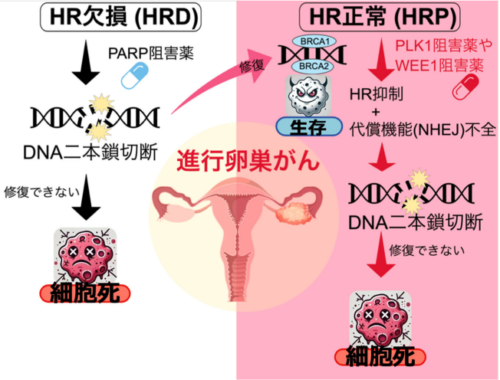
本研究の概要図
<関連情報>
- https://www.h.u-tokyo.ac.jp/press/20251225.html
- https://www.h.u-tokyo.ac.jp/press/__icsFiles/afieldfile/2025/12/25/release_20251225.pdf
- https://www.nature.com/articles/s41419-025-08324-2
PLK1またはWEE1阻害はBRCA1 / 2野生型高悪性度漿液性卵巣癌における相同組換え修復能を標的とする PLK1 or WEE1 inhibition targets homologous recombination repair proficiency in BRCA1/2 wild-type high-grade serous ovarian cancer
Qian Xi,Akiko Kunita,Miho Ogawa,Masanori Kawakami,Mirei Ka,Saeko Nagai,Anh Quynh Duong,Ayumi Taguchi,Kousuke Watanabe,Tomohiko Fukuda,Kenbun Sone,Aya Shinozaki-Ushiku,Tetsuo Ushiku,Yasushi Hirota,Hidenori Kage,Kazuhiro Katayama & Katsutoshi Oda
Cell Death & Disease Published:07 December 2025
DOI:https://doi.org/10.1038/s41419-025-08324-2
Abstract
High-grade serous ovarian cancer (HGSOC) is a poor prognostic disease, especially in BRCA1/2 wild-type (BRCA-WT) patients with homologous recombination (HR) proficiency. These patients often show limited response to both platinum-based chemotherapy and PARP inhibitors. HR and non-homologous end joining (NHEJ) are the two major DNA double-strand break (DSB) repair pathways. HR is a precise repair mechanism for DSBs but is limited to S and G2 phases. In contrast, NHEJ functions more broadly throughout the cell cycle, including G1. We investigated whether inhibiting the G2/M checkpoint kinases PLK1 or WEE1 individually could disrupt mitotic control and expose therapeutic vulnerabilities in BRCA-WT/HR-proficient HGSOC cells. We evaluated cell cycle–targeted strategies to overcome HR-proficient chemoresistance using either volasertib (a selective PLK1 inhibitor) or adavosertib (a potent WEE1 inhibitor) in BRCA-WT/HR-proficient and BRCA-mutant/HR-deficient HGSOC models. Both agents induced DNA damage, impaired HR repair (reduced RAD51 foci), and triggered mitotic catastrophe—a form of cell death caused by defective mitosis and unresolved DNA damage—in BRCA-WT cells. Volasertib caused polyploidy and abnormal spindle formation, indicating mitotic slippage and cytokinesis failure, whereas adavosertib abrogated the G2/M checkpoint, forcing premature mitotic entry. In contrast, BRCA-mutant cells were resistant to either volasertib or adavosertib, consistent with sustained and functional NHEJ activity. This resistance was restored by the pharmacological or genetic inhibition of DNA-PKcs (DNA-dependent protein kinase, catalytic subunit), a prominent component of NHEJ. Functional and xenograft models confirmed selective vulnerability of BRCA-WT tumors to either PLK1 or WEE1 inhibition. Our work highlights a mechanistic framework linking cell cycle checkpoint inhibition to DNA repair pathway selectivity, providing a rationale for targeting mitotic regulators in HR-proficient ovarian cancer—a subgroup with high clinical unmet need.