2026-01-07 ノースウェスタン大学
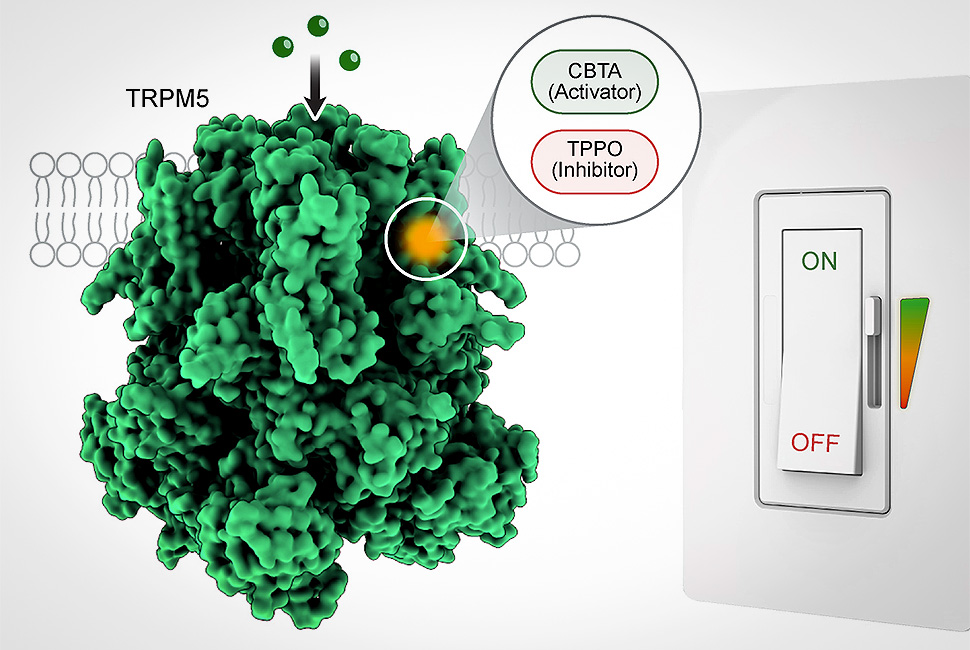
Northwestern scientists have discovered a “molecular switch” central to biological processes that connect taste, metabolism and gut health. The ability to fine-tune its activity could lead to new drugs that boost insulin release, curb cravings and reduce gut inflammation. Image by Juan Du/Wei Lu/Northwestern University
<関連情報>
- https://news.northwestern.edu/stories/2026/01/hidden-molecular-switch-controls-taste-metabolism-and-gut-function
- https://www.nature.com/articles/s41589-025-02097-7
- https://www.nature.com/articles/s41594-021-00607-4
TRPM5の活性化、調節、阻害は単一のアロステリック部位によって統合されている A single allosteric site merges activation, modulation and inhibition in TRPM5
Zheng Ruan,Junuk Lee,Yangyang Li,Ian J. Orozco,Juan Du & Wei Lü
Nature Chemical Biology Published:05 January 2026
DOI:https://doi.org/10.1038/s41589-025-02097-7
Abstract
TRPM5 is a Ca2+-activated monovalent cation channel essential for taste perception, insulin secretion and gastrointestinal chemosensation. Canonical TRPM5 activation requires Ca2+ binding at two distinct sites: an agonist site within the lower vestibule of the S1–S4 pocket in the transmembrane domain (CaTMD) and a modulatory site in the intracellular domain (CaICD) that tunes voltage dependence and agonist sensitivity. Here we characterize CBTA as a noncalcium agonist that binds to the upper vestibule of the S1–S4 pocket, directly above CaTMD. CBTA alone mimics the dual role of CaTMD and CaICD, merging agonist activation with voltage modulation. CBTA also renders TRPM5 supersensitive to Ca2+, synergistically hyperactivating the channel even at near-resting Ca2+ levels. We further demonstrate that the inhibitor triphenylphosphine oxide binds the same site but stabilizes a nonconductive state. These opposing effects reveal the upper S1–S4 pocket as a multifunctional regulatory hub integrating activation, inhibition and modulation in TRPM5.
TRPM5チャネルの構造は活性化と阻害のメカニズムを解明する Structures of the TRPM5 channel elucidate mechanisms of activation and inhibition
Zheng Ruan,Emery Haley,Ian J. Orozco,Mark Sabat,Richard Myers,Rebecca Roth,Juan Du & Wei Lü
Nature Structural & Molecular Biology Published:24 June 2021
DOI:https://doi.org/10.1038/s41594-021-00607-4
An Author Correction to this article was published on 20 July 2021
This article has been updated
Abstract
The Ca2+-activated TRPM5 channel plays essential roles in taste perception and insulin secretion. However, the mechanism by which Ca2+ regulates TRPM5 activity remains elusive. We report cryo-EM structures of the zebrafish TRPM5 in an apo closed state, a Ca2+-bound open state, and an antagonist-bound inhibited state. We define two novel ligand binding sites: a Ca2+ site (CaICD) in the intracellular domain and an antagonist site in the transmembrane domain (TMD). The CaICD site is unique to TRPM5 and has two roles: modulating the voltage dependence and promoting Ca2+ binding to the CaTMD site, which is conserved throughout TRPM channels. Conformational changes initialized from both Ca2+ sites cooperatively open the ion-conducting pore. The antagonist NDNA wedges into the space between the S1–S4 domain and pore domain, stabilizing the transmembrane domain in an apo-like closed state. Our results lay the foundation for understanding the voltage-dependent TRPM channels and developing new therapeutic agents.