2026-01-08 東京科学大学
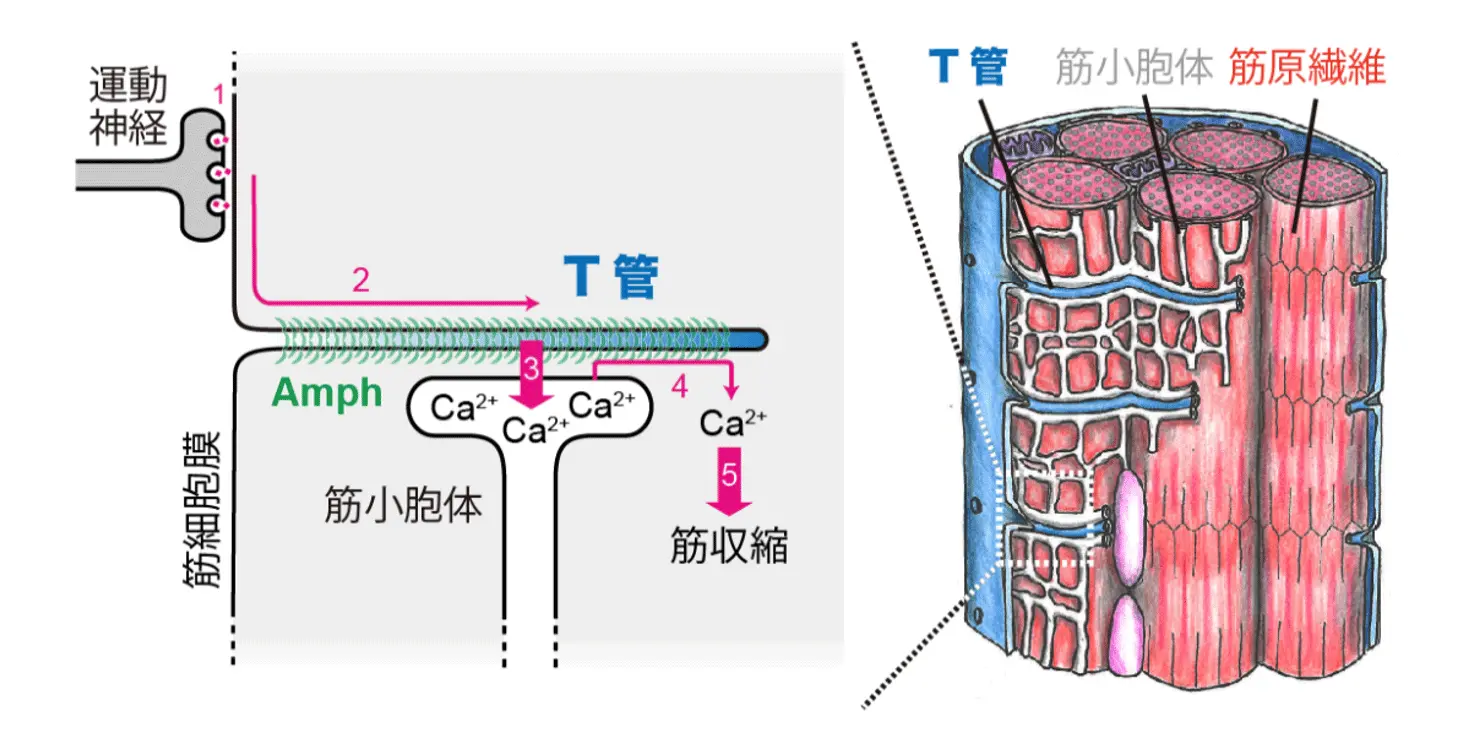
図1. T管を介した筋収縮の誘導(自著論文[参考文献1]を改変して作成したもの。ライセンス:CC BY 4.0.)
<関連情報>
線形ユビキチン化はAmphを介したT管の生合成を誘発する Linear ubiquitination triggers Amph-mediated T-tubule biogenesis
Kohei Kawaguchi, Yutaro Hama, Harunori Yoshikawa, Kohei Nishino, […] , and Naonobu Fujita
Science Advances Published:7 Jan 2026
DOI:https://doi.org/10.1126/sciadv.ady4934
Abstract
Transverse tubules (T-tubules) are invaginations of the muscle plasma membrane that facilitate rapid transmission of action potentials, ensuring synchronized muscle contraction. Despite their essential role in muscle physiology, the mechanisms underlying T-tubule formation remain elusive. Here, we identify LUBEL/RNF31, a ubiquitin E3 ligase responsible for linear (M1-linked) ubiquitination, as a key regulator of T-tubule biogenesis in Drosophila. Loss of LUBEL leads to Amphiphysin (Amph)–positive membrane sheets instead of tubular networks. The ubiquitin ligase activity of LUBEL and direct interaction with Amph, a BAR domain protein involved in membrane tubulation, are crucial for proper T-tubule morphology. LUBEL and M1-linked ubiquitin chains assemble into puncta on membranes through multivalent interactions, facilitating Amph-mediated tubulation. Notably, the Amph-LUBEL/RNF31 interaction is evolutionarily conserved across species, underscoring a fundamental role for linear ubiquitination in membrane remodeling. Our findings uncover an unexpected function of linear ubiquitination in membrane deformation driven by BAR proteins.