2026-01-22 東京科学大学
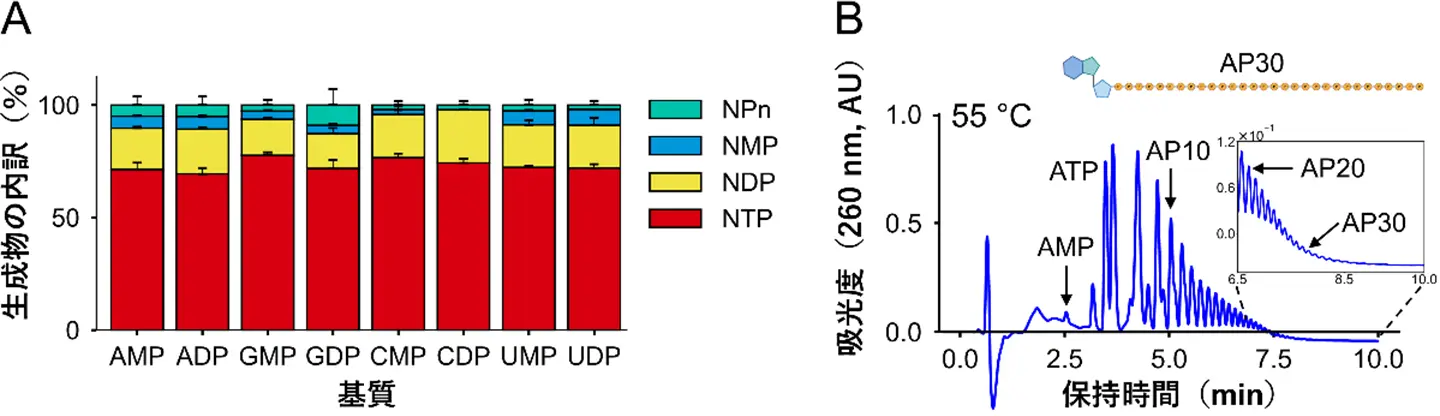
図1. MANによる酵素反応後の生成物。
(A)ポリリン酸と、8種のNMPまたはNDPを基質とし、37℃で反応させた後の生成物の内訳。いずれの基質も、約70%はNTPに変換された。
(B)アデノシン一リン酸を基質とし、55℃で反応させた後の生成物のクロマトグラム。各ピークは、リン酸基の数が1つずつ異なるアデノシンポリリン酸に対応する。AP30は、リン酸が30分子結合した分子を指す。
<関連情報>
ユニバーサルポリリン酸キナーゼがin vitro転写を促進する A universal polyphosphate kinase powers in vitro transcription
Ryusei Matsumoto,Takayoshi Watanabe,Eishin Yamazaki,Ako Kagawa,Liam M. Longo & Tomoaki Matsuura
Nature Communications Published:08 January 2026
DOI:https://doi.org/10.1038/s41467-025-68012-9
We are providing an unedited version of this manuscript to give early access to its findings. Before final publication, the manuscript will undergo further editing. Please note there may be errors present which affect the content, and all legal disclaimers apply.
Abstract
Polyphosphate kinases (PPKs) catalyze phosphoryl transfer between polyphosphates and nucleotides. Polyphosphates are a cost-effective source of phosphorylating power, making PPKs attractive enzymes for nucleotide production. However, at present, applications that require the simultaneous utilization of diverse nucleotides are not possible due to the restricted substrate profiles of PPKs. Here, we present a universal PPK capable of efficiently phosphorylating all eight common ribonucleotides (purines and pyrimidines, monophosphates and diphosphates) to triphosphates. Under optimal conditions, ~70% triphosphate conversion was observed for each substrate. To demonstrate the biotechnological potential of a universal PPK, we developed a one-pot assay for PPK-powered in vitro transcription. Primitive biology likely relied on enzyme promiscuity to support nascent metabolism with a compact proteome. This work highlights how applying the same principle to synthetic biology can facilitate the construction of complex in vitro reaction systems.


