2026-01-28 韓国基礎科学研究院(IBS)
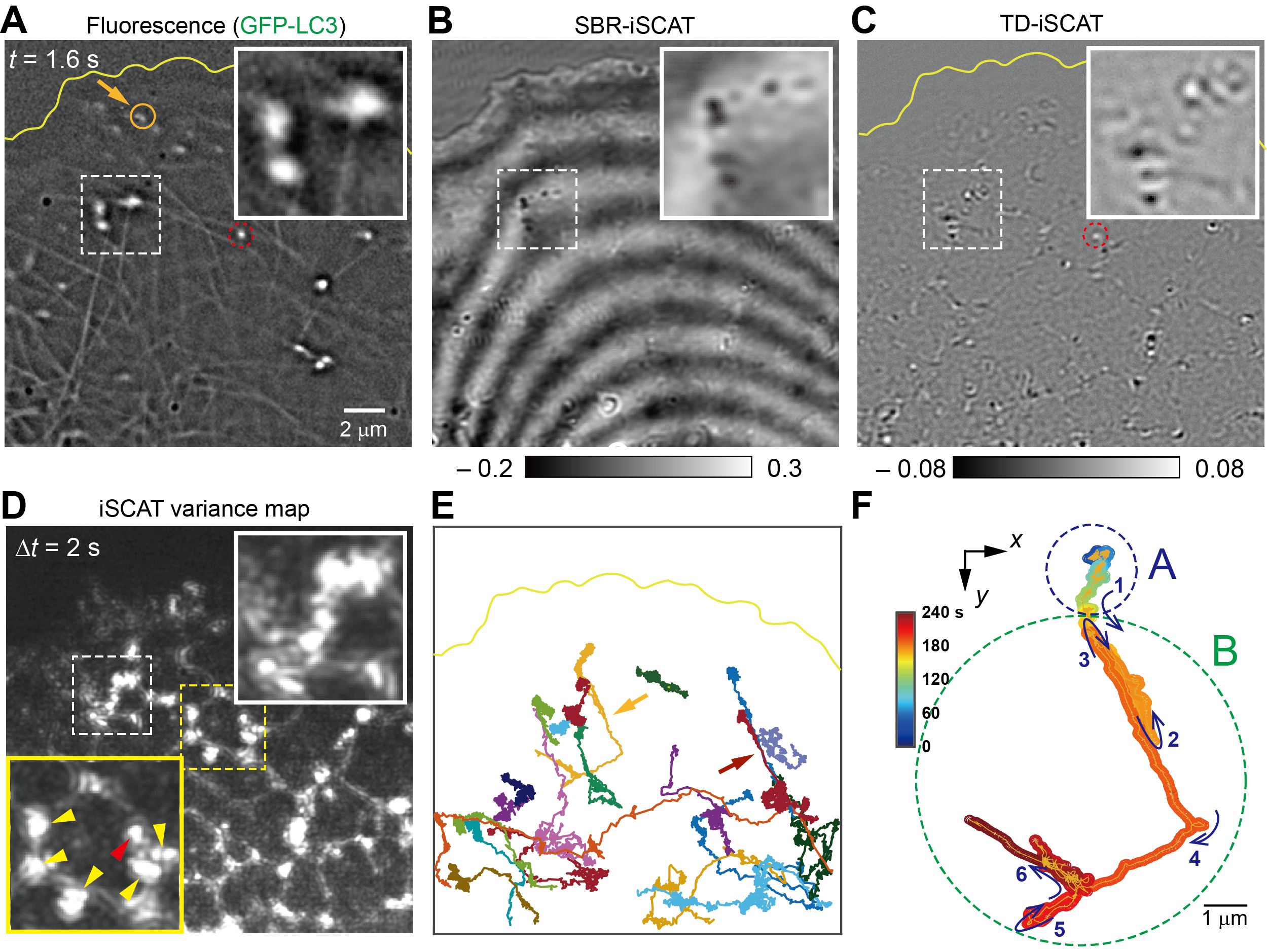
Figure 1. Diverse movements of autophagosomes within the crowded cytoplasm of a living cell
By combining fluorescence imaging with DySLIM, the researchers simultaneously visualized autophagosomes, microtubules, and the ER network, capturing organelle hand-off events with millisecond temporal resolution and nanometer-scale spatial precision.
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=26517
- https://pubs.acs.org/doi/10.1021/acsnano.5c15844
細胞小器官ハンドオフポータルのナノスコピーにより、小胞体リモデリングと微小管輸送の直接的な連携が明らかに Nanoscopy of Organelle Handoff Portals Reveals Direct Coupling between Endoplasmic Reticulum Remodeling and Microtubule-Based Transport
Jin-Sung Park,Il-Buem Lee,Hyeon-Min Moon,Hyeonjun Jeon,MinHyeong Lee,Chungho Kim,Seok-Cheol Hong,and Minhaeng Cho
ACS Nano Published: January 20, 2026
DOI:https://doi.org/10.1021/acsnano.5c15844
Abstract
How biosynthetic organelles leave the endoplasmic reticulum (ER) and engage with microtubule tracks remains a central question. Combining interferometric scattering with fluorescence nanoscopy, we tracked nanometer-scale handoff events in living cells. ER-derived organelles undergo biased diffusion along ER tubules toward nearby microtubules. ER three-way junctions function as nanoscopic hubs where a cargo pauses, contacts multiple microtubules, and then launches onto a track for long-range travel. During this process, the ER maintains a membrane tether to the departing cargo, extending its tubules and forming new junctions, thereby coupling internetwork transfer with membrane morphogenesis. These observations reveal an integrated mechanism that links organelle biogenesis, directional trafficking, and continual ER and cellular remodeling, underscoring the ER’s active role in steering transport and repurposing its own output. More broadly, this single-label, dual-mode nanoscopy provides a minimally perturbative, high-speed, and broadly applicable platform for probing the nanoscale dynamics of diverse organelles and cytoskeletal processes in the crowded intracellular environment.


