2026-02-09 レンセラー工科大学
<関連情報>
- https://news.rpi.edu/2026/02/09/research-reveals-how-brain-can-increase-resilience-disease
- https://pubs.acs.org/doi/10.1021/jacs.5c15573
- https://www.nature.com/articles/s41591-023-02318-3
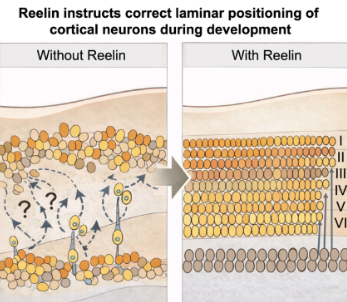
N-硫酸化ヘパラン硫酸は共受容体としてリーリンシグナル伝達を促進する N-Sulfated Heparan Sulfate Promotes Reelin Signaling as a Co-receptor
Lin Pan,Xuehong Song,Guowei Su,Lauren A Gandy,Biqin Fang,Mason Buttaci,James Gibson,Ke Xia,Fuming Zhang,Jian Liu,Lianchun Wang,Sally Temple,and Chunyu Wang
Journal of the American Chemical Society Published: December 8, 2025
DOI:https://doi.org/10.1021/jacs.5c15573
Abstract
Heparan sulfate (HS) plays a central role in signal transduction, while Reelin is an essential signaling protein in both the developing and adult brain. A Reelin COLBOS variant was recently discovered with enhanced HS binding and resilience against autosomal dominant Alzheimer’s disease (ADAD), underscoring the importance of Reelin–HS interactions. However, the glycan determinants of Reelin–HS interactions have not been well-characterized, which we systematically investigated here. Surface plasmon resonance (SPR) showed that full length Reelin binds HS with high affinity (KD = 17 ± 5 nM), which is enhanced by the COLBOS variant (KD = 10 ± 2 nM). Competition SPR and glycan array studies further revealed that HS N-sulfation is critical for Reelin–HS binding, consistent with Haddock modeling. In cell surface binding assays, heparinase treatment, which degrades HS, or the knockout of a key HS N-sulfation enzyme (NDST1) significantly reduced Reelin attachment. Functionally, a cellular split-luciferase assay showed that heparinase treatment or adding heparin in culture medium reduces Reelin-induced ApoER2 dimerization, demonstrating that HS is a coreceptor for Reelin receptor activation. In contrast, N-desulfated heparin does not inhibit Reelin receptor dimerization. Our work establishes HS as a coreceptor for Reelin signaling and N-sulfation as a key glycan determinant of Reelin–HS recognition. Our work provides mechanistic insights into diverse neurodevelopmental and neurodegenerative diseases associated with Reelin signaling and suggests novel therapeutic strategies targeting HS sulfation.
リーリン-コルボスヘテロ接合性男性における常染色体優性アルツハイマー病に対する回復力 Resilience to autosomal dominant Alzheimer’s disease in a Reelin-COLBOS heterozygous man
Francisco Lopera,Claudia Marino,Anita S. Chandrahas,Michael O’Hare,Nelson David Villalba-Moreno,David Aguillon,Ana Baena,Justin S. Sanchez,Clara Vila-Castelar,Liliana Ramirez Gomez,Natalia Chmielewska,Gabriel M. Oliveira,Jessica Lisa Littau,Kristin Hartmann,Kyungeun Park,Susanne Krasemann,Markus Glatzel,Dorothee Schoemaker,Lucia Gonzalez-Buendia,Santiago Delgado-Tirado,Said Arevalo-Alquichire,Kahira L. Saez-Torres,Dhanesh Amarnani,Leo A. Kim,… Yakeel T. Quiroz
Nature Medicine Published:15 May 2023
DOI: https://doi.org/10.1038/s41591-023-02318-3
Abstract
We characterized the world’s second case with ascertained extreme resilience to autosomal dominant Alzheimer’s disease (ADAD). Side-by-side comparisons of this male case and the previously reported female case with ADAD homozygote for the APOE3 Christchurch (APOECh) variant allowed us to discern common features. The male remained cognitively intact until 67 years of age despite carrying a PSEN1-E280A mutation. Like the APOECh carrier, he had extremely elevated amyloid plaque burden and limited entorhinal Tau tangle burden. He did not carry the APOECh variant but was heterozygous for a rare variant in RELN (H3447R, termed COLBOS after the Colombia–Boston biomarker research study), a ligand that like apolipoprotein E binds to the VLDLr and APOEr2 receptors. RELN-COLBOS is a gain-of-function variant showing stronger ability to activate its canonical protein target Dab1 and reduce human Tau phosphorylation in a knockin mouse. A genetic variant in a case protected from ADAD suggests a role for RELN signaling in resilience to dementia.