2026-02-16 インペリアル・カレッジ・ロンドン(ICL)
<関連情報>
- https://www.imperial.ac.uk/news/articles/2026/ayahuasca-compound-has-significant-and-lasting-effect-on-depression-/
- https://www.nature.com/articles/s41591-025-04154-z
大うつ病性障害に対する短期作用型サイケデリック介入:第IIa相ランダム化プラセボ対照試験 A short-acting psychedelic intervention for major depressive disorder: a phase IIa randomized placebo-controlled trial
David Erritzoe,Tommaso Barba,Tiffanie Benway,Zelah Joel,Meghan Good,Marie Layzell,Michelle Baker Jones,Graham Campbell,Ashleigh Murphy-Beiner,Peter Rands,Malcolm Boyce,Helen Topping,Brandon Weiss,Christopher Timmermann,David Nutt,Robin Carhart-Harris,Carol Routledge & Ellen James
Nature Medicine Published:16 February 2026
DOI:https://doi.org/10.1038/s41591-025-04154-z
Abstract
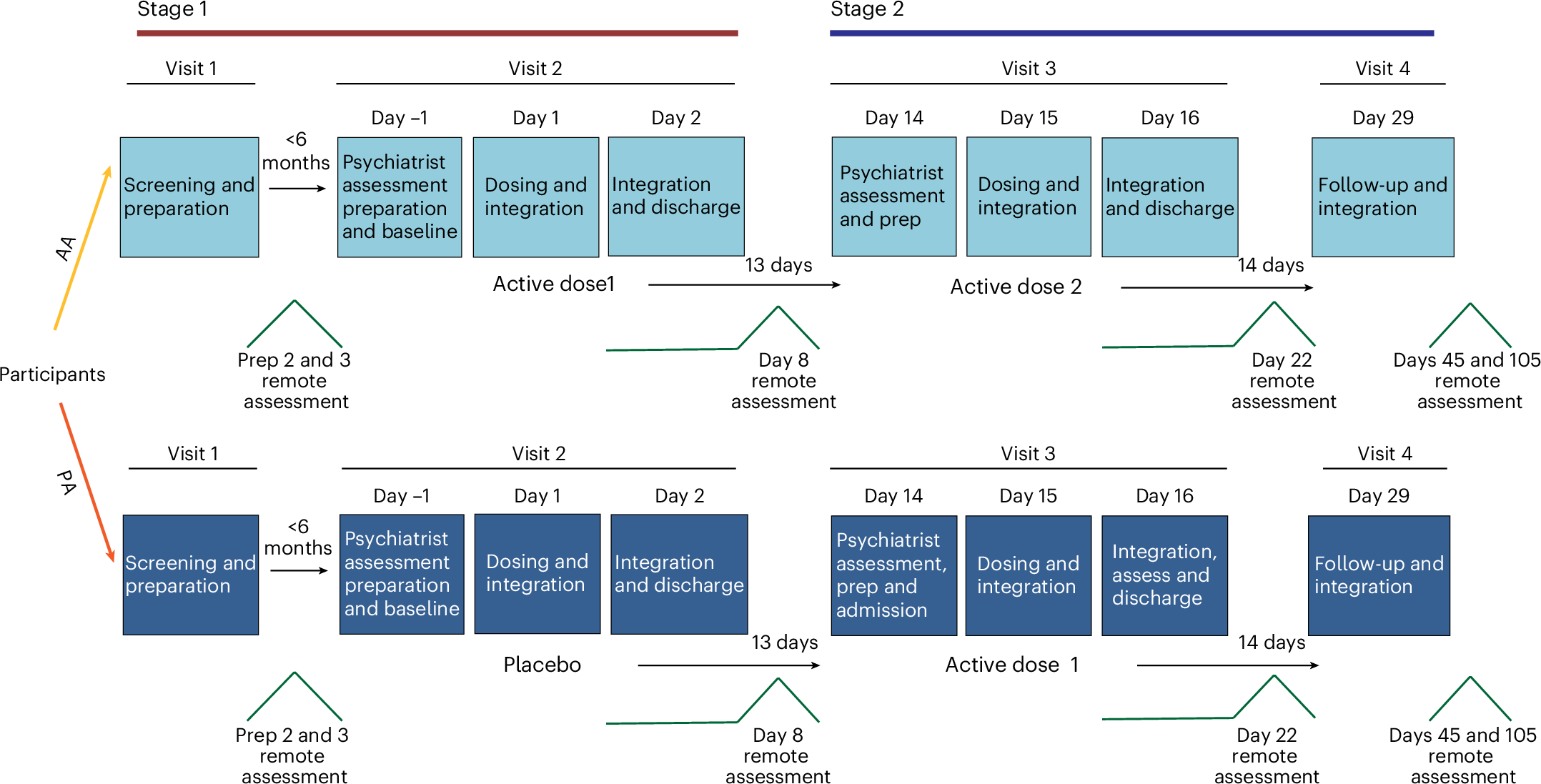
Major depressive disorder (MDD) is a leading cause of disability worldwide, yet many patients have inadequate responses to current treatments. Dimethyltryptamine (DMT), a serotonergic psychedelic with rapid onset and short duration, shows promise as a potential antidepressant (AD), although clinical evidence in MDD remains limited. We conducted a phase IIa, double-blind, placebo-controlled, randomized clinical trial to evaluate the efficacy and safety of intravenous DMT (SPL026; DMT fumarate) in adults with moderate-to-severe MDD. Participants received a single 21.5-mg dose of DMT or placebo infused over 10 min, along with supportive psychotherapeutic support, followed by a 2-week assessment. A subsequent open-label phase offered all participants a second DMT dose. The primary outcome was the change in Montgomery–Åsberg Depression Rating Scale (MADRS) at 2 weeks. Secondary outcomes included response (≥50% reduction in MADRS score) and remission (MADRS ≤ 10). A total of 34 participants were randomized, 17 to placebo–active and 17 to active–active. At 2 weeks, the DMT group showed a significantly greater reduction in MADRS score than placebo (mean difference = -7.35; 95% CI = -13.62 to -1.08; P = 0.023). In the open-label phase, AD effects persisted up to 3 months, with no significant differences between those who received one versus two doses. Adverse events were mostly mild to moderate, commonly infusion site pain, nausea and transient anxiety. No serious adverse events occurred. A single dose of DMT with psychotherapeutic support produced a rapid, significant reduction in depressive symptoms, sustained up to 3 months. The treatment was well-tolerated and safe. ClinicalTrials.gov registration: NCT04673383.