「いずれは別のエマージェンシー病が発生する」 ‘Eventually there will be another emergent disease’
2022-03-22 ノースウェスタン大学
新しいナノ粒子ベースのCOVID-19ワクチンを1回投与するだけで、現在臨床で使用されているワクチンと同程度の免疫反応を動物にもたらすことができました。ノースウェスタン大学の研究者たちは、わずかな変更で、同じワクチン・プラットフォームが他の感染症も標的にできることを期待している。
新しい研究では、COVID-19の原因となるSARS-CoV-2ウイルスの致死量を投与したところ、タンパク質ベースの免疫を受けたマウスの100%が生存した。また、SARS-CoV-2への曝露により肺に損傷を受けたマウスは一匹もいなかった。このナノ粒子ワクチンを投与しなかったマウスは、14日間の試験ですべて死亡した。
この結果は、今週の米国科学アカデミー紀要に掲載され、ウイルス感染から身を守るために開発された初の球状核酸(SNA)ワクチンの構造-機能関係の概要が明らかになりました。
<関連情報>
- https://news.northwestern.edu/stories/2022/03/sna-covid-vaccine/
- https://www.pnas.org/doi/pdf/10.1073/pnas.2119093119
感染症ワクチンプラットフォームとしての球状核酸 Spherical nucleic acids as an infectious disease vaccine platform
Michelle H. Teplenskya,b,1, Max E. Distlera,b,1, Caroline D. Kusmierza,b , Michael Evangelopoulosc , Haley Gulad , Derek Ellid , Anastasia Tomatsidoud , Vlad Nicolaescud , Ian Gelardene , Anjana Yeldandie , Daniel Batllef , Dominique Missiakasd , and Chad A. Mirkina,b,c,2 Contributed by Chad A. Mirkin; received October 18, 2021; accepted January 5, 2022; reviewed by Daniel Anderson and Molly Stevens
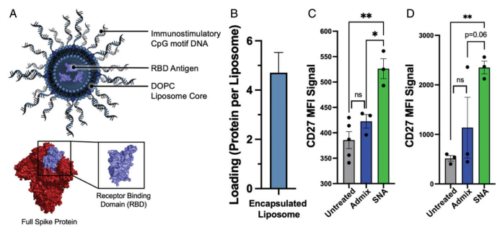
Despite recent efforts demonstrating that organization and presentation of vaccine components are just as important as composition in dictating vaccine efficacy, antiviral vaccines have long focused solely on the identification of the immunological target. Herein, we describe a study aimed at exploring how vaccine component presentation in the context of spherical nucleic acids (SNAs) can be used to elicit and maximize an antiviral response. Using COVID-19 as a topical example of an infectious disease with an urgent need for rapid vaccine development, we designed an antiviral SNA vaccine, encapsulating the receptor-binding domain (RBD) subunit into a liposome and decorating the core with a dense shell of CpG motif toll-like receptor 9 agonist oligonucleotides. This vaccine induces memory B cell formation in human cells, and in vivo administration into mice generates robust binding and neutralizing antibody titers. Moreover, the SNA vaccine outperforms multiple simple mixtures incorporating clinically employed adjuvants. Through modular changes to SNA structure, we uncover key relationships and proteomic insights between adjuvant and antigen ratios, concepts potentially translatable across vaccine platforms and disease models. Importantly, when humanized ACE2 transgenic mice were challenged in vivo against a lethal live virus, only mice that received the SNA vaccine had a 100% survival rate and lungs that were clear of virus by plaque analysis. This work underscores the potential for SNAs to be implemented as an easily adaptable and generalizable platform to fight infectious disease and demonstrates the importance of structure and presentation in the design of next-generation antiviral vaccines.