2022-03-14 テキサス大学オースティン校(UTオースティン)
<関連情報>
- https://news.utexas.edu/2022/03/14/new-type-of-vaccines-could-help-against-more-respiratory-illnesses/
- https://www.nature.com/articles/s41467-022-28931-3
プレフュージョンで安定化したヒトメタニューモウイルス融合タンパク質の構造に基づく設計 Structure-based design of prefusion-stabilized human metapneumovirus fusion proteins
Ching-Lin Hsieh,Scott A. Rush,Concepcion Palomo,Chia-Wei Chou,Whitney Pickens,Vicente Más &Jason S. McLellan
Published: 14 March 2022
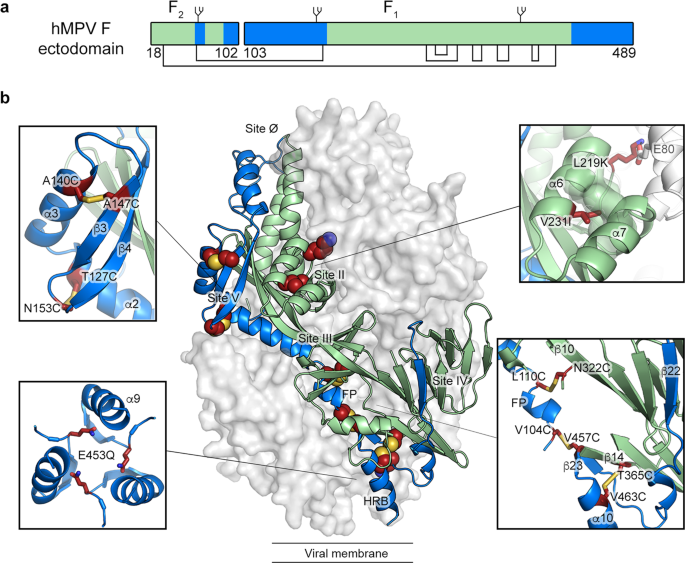
Abstract
The human metapneumovirus (hMPV) fusion (F) protein is essential for viral entry and is a key target of neutralizing antibodies and vaccine development. The prefusion conformation is thought to be the optimal vaccine antigen, but previously described prefusion F proteins expressed poorly and were not well stabilized. Here, we use structures of hMPV F to guide the design of 42 variants containing stabilizing substitutions. Through combinatorial addition of disulfide bonds, cavity-filling substitutions, and improved electrostatic interactions, we describe a prefusion-stabilized F protein (DS-CavEs2) that expresses at 15 mg/L and has a melting temperature of 71.9 °C. Crystal structures of two prefusion-stabilized hMPV F variants reveal that antigenic surfaces are largely unperturbed. Importantly, immunization of mice with DS-CavEs2 elicits significantly higher neutralizing antibody titers against hMPV A1 and B1 viruses than postfusion F. The improved properties of DS-CavEs2 will advance the development of hMPV vaccines and the isolation of therapeutic antibodies.