ポイントオブケア診断のための臨床試料の直接・高感度分析 Direct and sensitive analysis of clinical samples for point-of-care diagnostics
2022-05-18 大韓民国・基礎科学研究院(IBS)
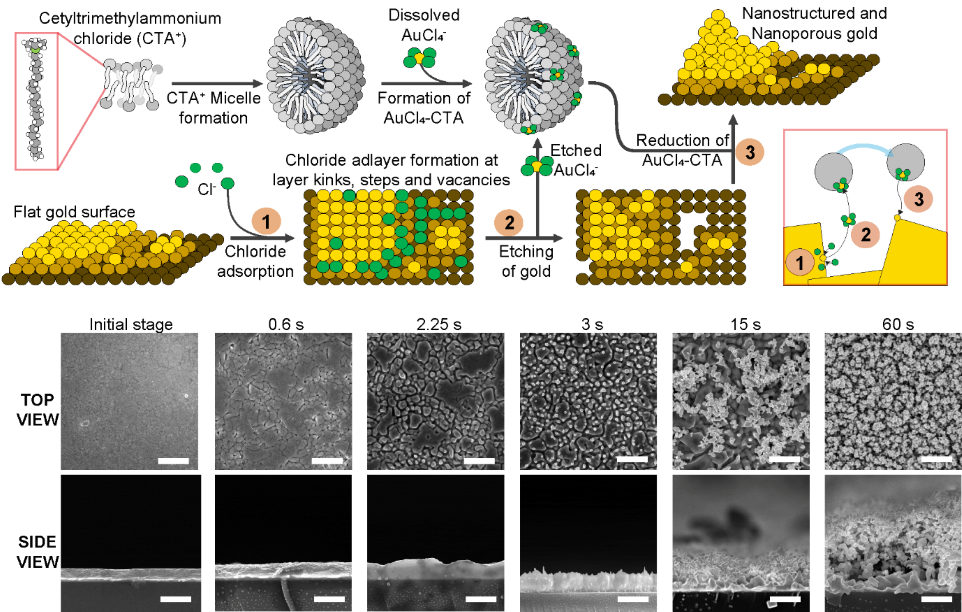 Mechanism to generate nanostructured and nanoporous gold surfaces based on the preferential etch and deposition of the substrate using a surfactant that forms micelles in solution, sodium chloride, and a gold salt. Applying electric pulses, first, chloride is adsorbed on the surface, then gold is etched away but captured by the surfactant micelles. Finally, it is redeposited on the substrate growing the nanostructures in the process. At the bottom, scanning electron micrographs show the formation of nanostructures and nanopores on the surface throughout the process.
Mechanism to generate nanostructured and nanoporous gold surfaces based on the preferential etch and deposition of the substrate using a surfactant that forms micelles in solution, sodium chloride, and a gold salt. Applying electric pulses, first, chloride is adsorbed on the surface, then gold is etched away but captured by the surfactant micelles. Finally, it is redeposited on the substrate growing the nanostructures in the process. At the bottom, scanning electron micrographs show the formation of nanostructures and nanopores on the surface throughout the process.
しかし、このような機器の開発には、病気や感染症などのバイオマーカーは、サンプル中にごく微量しか存在しないため、高感度な検出技術の開発が課題となっていた。バイオセンサーの表面積を増やせば感度は向上するが、表面はすぐに目詰まりを起こし、汚れて使えなくなる傾向がある。
基礎科学研究所(IBS)ソフト&リビングマターセンターのCHO, Yoon-Kyoung教授率いるチームは、ナノ構造およびナノ多孔質表面を生成する方法を用いて、最近バイオセンサーを開発しました。この複合戦略は、センサーに前例のない感度をもたらすだけでなく、タンパク質による汚れに耐性を持たせるものである。
これまで、このようなナノ構造・ナノポーラス基板を用いた電極を確実に生成する方法は知られていなかったが、研究チームは、このような材料を簡単に生成する方法を報告した。その仕組みは、塩化ナトリウムと溶液中でミセルを形成できる界面活性剤の存在下、平坦な金表面に電気パルスを印加することに基づいている。
<関連情報>
- https://www.ibs.re.kr/cop/bbs/BBSMSTR_000000000738/selectBoardArticle.do?nttId=21306&pageIndex=1
- https://onlinelibrary.wiley.com/doi/abs/10.1002/adma.202200981
原液生体試料中のバイオマーカーを高感度に電気化学的に検出する「SEEDING」を開発 SEEDING to Enable Sensitive Electrochemical Detection of Biomarkers in Undiluted Biological Samples
Jonathan Sabaté del Río,Hyun-Kyung Woo,Juhee Park,Hong Koo Ha,Jae-Ryong Kim,Yoon-Kyoung Cho
Advanced Materials Published: 15 April 2022
DOI:https://doi.org/10.1002/adma.202200981
Abstract
Electrochemical biosensors have shown great potential for simple, fast, and cost-effective point-of-care diagnostic tools. However, direct analysis of complex biological fluids such as plasma has been limited by the loss of sensitivity caused by biofouling. By increasing the surface area, the nanostructured electrode can improve detection sensitivity. However, like a double-edged sword, a large surface area increases the nonspecific adsorption of contaminating proteins. The use of nanoporous structures may prevent fouling proteins. However, there is no straightforward approach for creating nanostructured and nanoporous surfaces compatible with microfabricated thin-film electrodes. Herein, the preferential etching of chloride and surfactant-assisted anisotropic gold reduction to create homogeneous, nanostructured, and nanoporous gold electrodes is demonstrated, yielding a 190 ± 20 times larger surface area within a minute without using templates. This process, “surfactant-based electrochemical etch-deposit interplay for nanostructure/nanopore growth” (SEEDING), on electrodes enhances the sensitivity and antibiofouling capabilities of amperometric biosensors, enabling direct analysis of tumor-derived extracellular vesicles (tEVs) in complex biofluids with a limit of detection of 300 tEVs µL-1 from undiluted plasma and good discrimination between patients with prostate cancer from healthy ones with an area under the curve of 0.91 in urine and 0.90 in plasma samples.