2024-01-09 スイス連邦工科大学ローザンヌ校(EPFL)
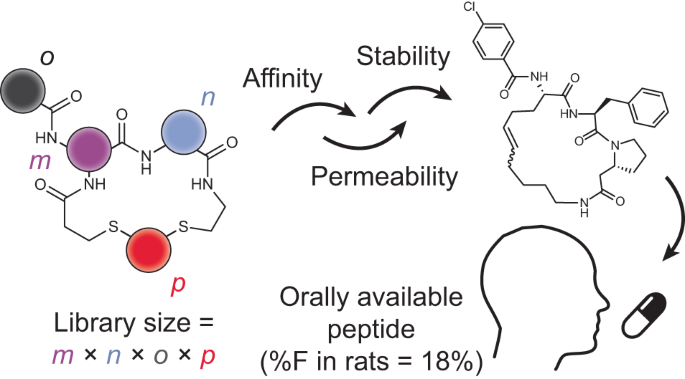
◆研究では、血液凝固における中心的な役割を果たす酵素であるトロンビンをターゲットにし、安定性を持つ環状ペプチドを生成するための新しい合成戦略を開発しました。ラットでの試験では、これらのペプチドが口腔内での生物学的利用度が最大18%まで達することが示され、通常は2%以下である口腔内投与された環状ペプチドにおいて、18%に増加させることが可能となりました。この成果により、従来の口腔内薬では難しかった疾患への治療の可能性が広がり、医学の未解決の分野での重要な進展が期待されます。
<関連情報>
- https://actu.epfl.ch/news/oral-peptides-a-new-era-in-drug-development/
- https://www.nature.com/articles/s41589-023-01496-y
経口で生物学的に利用可能な小さな環状ペプチドのデノボ開発 De novo development of small cyclic peptides that are orally bioavailable
Manuel L. Merz,Sevan Habeshian,Bo Li,Jean-Alexandre G. L. David,Alexander L. Nielsen,Xinjian Ji,Khaled Il Khwildy,Maury M. Duany Benitez,Phoukham Phothirath & Christian Heinis
Nature Chemical Biology Published:28 December 2023
DOI:https://doi.org/10.1038/s41589-023-01496-y
Abstract
Cyclic peptides can bind challenging disease targets with high affinity and specificity, offering enormous opportunities for addressing unmet medical needs. However, as with biological drugs, most cyclic peptides cannot be applied orally because they are rapidly digested and/or display low absorption in the gastrointestinal tract, hampering their development as therapeutics. In this study, we developed a combinatorial synthesis and screening approach based on sequential cyclization and one-pot peptide acylation and screening, with the possibility of simultaneously interrogating activity and permeability. In a proof of concept, we synthesized a library of 8,448 cyclic peptides and screened them against the disease target thrombin. Our workflow allowed multiple iterative cycles of library synthesis and yielded cyclic peptides with nanomolar affinities, high stabilities and an oral bioavailability (%F) as high as 18% in rats. This method for generating orally available peptides is general and provides a promising push toward unlocking the full potential of peptides as therapeutics.