2024-04-22 パデュー大学
<関連情報>
- https://www.purdue.edu/newsroom/releases/2024/Q2/genetically-engineering-a-treatment-for-incurable-brain-tumors.html
- https://www.nature.com/articles/s41467-024-46343-3
synNotchでプログラムされたiPSC由来のNK細胞は、膠芽腫治療のためにTIGITとCD73の活性を奪う synNotch-programmed iPSC-derived NK cells usurp TIGIT and CD73 activities for glioblastoma therapy
Kyle B. Lupo,Xue Yao,Shambhavi Borde,Jiao Wang,Sandra Torregrosa-Allen,Bennett D. Elzey,Sagar Utturkar,Nadia A. Lanman,MacKenzie McIntosh & Sandro Matosevic
Nature Communications Published:01 March 2024
DOI:https://doi.org/10.1038/s41467-024-46343-3
Abstract
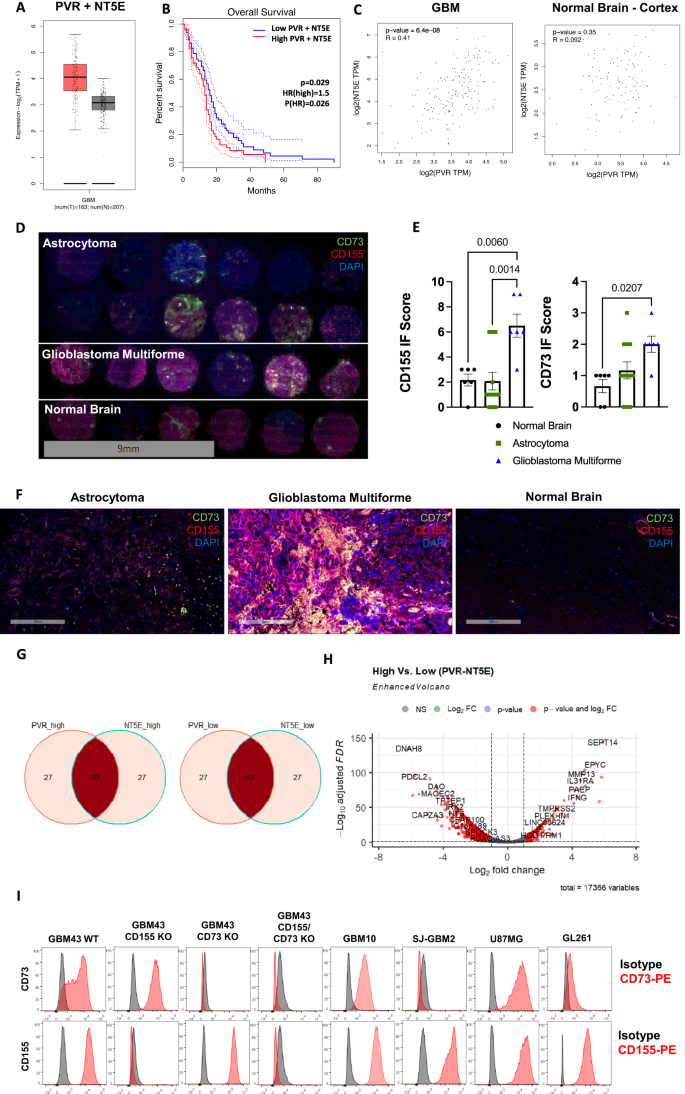
Severe heterogeneity within glioblastoma has spurred the notion that disrupting the interplay between multiple elements on immunosuppression is at the core of meaningful anti-tumor responses. T cell immunoreceptor with Ig and ITIM domains (TIGIT) and its glioblastoma-associated antigen, CD155, form a highly immunosuppressive axis in glioblastoma and other solid tumors, yet targeting of TIGIT, a functionally heterogeneous receptor on tumor-infiltrating immune cells, has largely been ineffective as monotherapy, suggesting that disruption of its inhibitory network might be necessary for measurable responses. It is within this context that we show that the usurpation of the TIGIT - CD155 axis via engineered synNotch-mediated activation of induced pluripotent stem cell-derived natural killer (NK) cells promotes transcription factor-mediated activation of a downstream signaling cascade that results in the controlled, localized blockade of CD73 to disrupt purinergic activity otherwise resulting in the production and accumulation of immunosuppressive extracellular adenosine. Such “decoy” receptor engages CD155 binding to TIGIT, but tilts inhibitory TIGIT/CD155 interactions toward activation via downstream synNotch signaling. Usurping activities of TIGIT and CD73 promotes the function of adoptively transferred NK cells into intracranial patient-derived models of glioblastoma and enhances their natural cytolytic functions against this tumor to result in complete tumor eradication. In addition, targeting both receptors, in turn, reprograms the glioblastoma microenvironment via the recruitment of T cells and the downregulation of M2 macrophages. This study demonstrates that TIGIT/CD155 and CD73 are targetable receptor partners in glioblastoma. Our data show that synNotch-engineered pluripotent stem cell-derived NK cells are not only effective mediators of anti-glioblastoma responses within the setting of CD73 and TIGIT/CD155 co-targeting, but represent a powerful allogeneic treatment option for this tumor.


