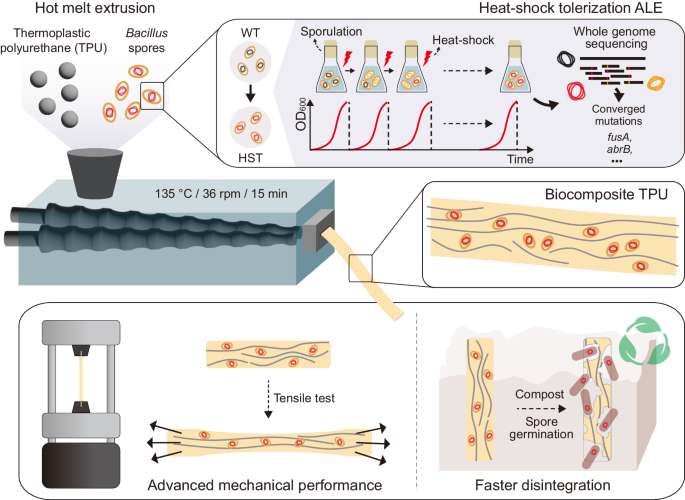
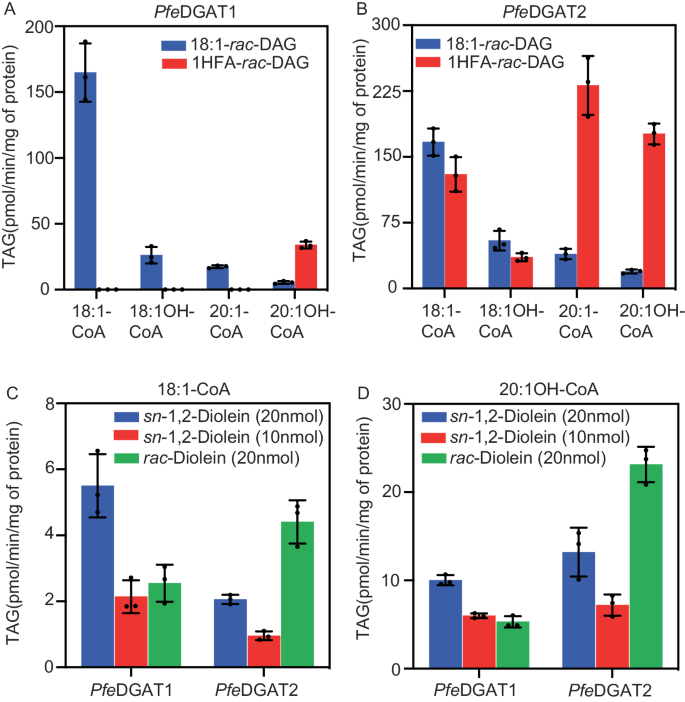
2024-04-30 パシフィック・ノースウェスト国立研究所(PNNL)

The increasing availability of high-resolution characterization of natural organic matter (OM) data has shifted the paradigm of lumped descriptions of OM components and potential microbial activities.
(Photo by Glen Carrie | Unsplash)
<関連情報>
- https://www.pnnl.gov/publications/unraveling-microbial-redox-dynamics
- https://www.sciencedirect.com/science/article/abs/pii/S0038071724000531?via%3Dihub
異なる電子受容体間における有機物分解の熱力学的制御 Thermodynamic control on the decomposition of organic matter across different electron acceptors
Jianqiu Zheng, Timothy D. Scheibe, Kristin Boye, Hyun-Seob Song
Soil Biology and Biochemistry Available online:12 March 2024
DOI:https://doi.org/10.1016/j.soilbio.2024.109364
Highlights
•A generalized model flexibly integrates organic matter (OM) chemistry and electron acceptors.
•The energy status of OM substrates leads to deviations from a fixed hierarchy of redox pathways.
•Thermodynamic model predicts key microbial growth parameters such as carbon use efficiency.
•Bioenergetics integrates stoichiometric and kinetic constraints on OM decomposition.
Abstract
The increasing availability of high-resolution characterization of natural organic matter (OM) data has shifted the paradigm of lumped descriptions of OM components and potential microbial activities. Our recent development of a substrate-explicit thermodynamic model uniquely enables incorporating complex OM pools to formulate biogeochemical reaction models based on their elemental compositions. While this previous work facilitates prediction of aerobic respiration of complex OM, it is equally imperative to consider the role of non-oxygenic electron acceptors in regulating OM turnover and the fate of carbon. In this study, we significantly expand our previous model by flexibly incorporating both detailed OM chemistry and electron acceptors other than oxygen. Our modeling analysis has revealed substantial variations in the energy status of OM molecules across different soils, which drive the co-occurrence of different electron-accepting processes. We demonstrated the effectiveness of the proposed model using a consistency check with experimental data. Through systematic evaluation of the impact of diverse chemical inputs (both electron donors and acceptors) on OM decomposition, the new model also revealed how key microbial growth parameters such as carbon use efficiency (CUE) and reaction rates vary across different electron-accepting processes. Our model provides a unified framework integrating thermodynamic and kinetic constraints on microbial metabolic activities. It complements traditional kinetic models, which are often designed solely to capture mass fluxes. We conclude that thermodynamic modeling emerges as a powerful tool for describing the mechanisms underlying the interplay between microbial growth and OM chemistry and cycling across different electron acceptors, enhancing our ability to project complex ecosystem behaviors in dynamic environments.