2024-07-19 スイス連邦工科大学ローザンヌ校(EPFL)
<関連情報>
- https://actu.epfl.ch/news/enhancing-breast-cancer-diagnosis-and-treatment/
- https://www.nature.com/articles/s41523-024-00665-z
EMBERは、独立した乳癌トランスクリプトームデータセットの統一された空間を作り出し、精密腫瘍学を可能にする EMBER creates a unified space for independent breast cancer transcriptomic datasets enabling precision oncology
Carlos Ronchi,Syed Haider & Cathrin Brisken
NPJ Breast Cancer Published:09 July 2024
DOI:https://doi.org/10.1038/s41523-024-00665-z
Abstract
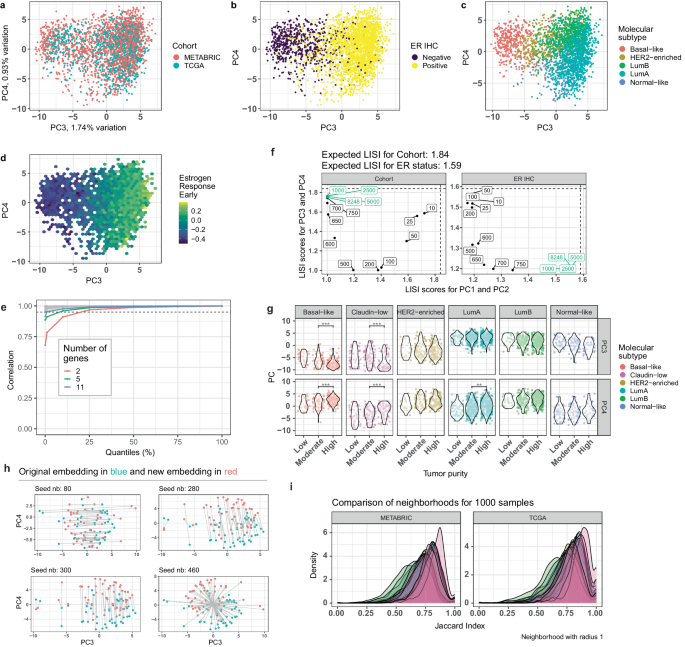
Transcriptomics has revolutionized biomedical research and refined breast cancer subtyping and diagnostics. However, wider use in clinical practice is hampered for a number of reasons including the application of transcriptomic signatures as single sample predictors. Here, we present an embedding approach called EMBER that creates a unified space of 11,000 breast cancer transcriptomes and predicts phenotypes of transcriptomic profiles on a single sample basis. EMBER accurately captures the five molecular subtypes. Key biological pathways, such as estrogen receptor signaling, cell proliferation, DNA repair, and epithelial-mesenchymal transition determine sample position in the space. We validate EMBER in four independent patient cohorts and show with samples from the window trial, POETIC, that it captures clinical responses to endocrine therapy and identifies increased androgen receptor signaling and decreased TGFβ signaling as potential mechanisms underlying intrinsic therapy resistance. Of direct clinical importance, we show that the EMBER-based estrogen receptor (ER) signaling score is superior to the immunohistochemistry (IHC) based ER index used in current clinical practice to select patients for endocrine therapy. As such, EMBER provides a calibration and reference tool that paves the way for using RNA-seq as a standard diagnostic and predictive tool for ER+ breast cancer.