2024-09-02 バーミンガム大学
<関連情報>
- https://www.birmingham.ac.uk/news/2024/blood-stem-cell-breakthrough-could-transform-bone-marrow-transplants
- https://www.nature.com/articles/s41587-024-02360-7
- https://www.nature.com/articles/s41467-023-35910-9
ヒト人工多能性幹細胞から分化した多系統造血細胞の長期生着 Long-term engrafting multilineage hematopoietic cells differentiated from human induced pluripotent stem cells
Elizabeth S. Ng,Gulcan Sarila,Jacky Y. Li,Hasindu S. Edirisinghe,Ritika Saxena,Shicheng Sun,Freya F. Bruveris,Tanya Labonne,Nerida Sleebs,Alexander Maytum,Raymond Y. Yow,Chantelle Inguanti,Ali Motazedian,Vincenzo Calvanese,Sandra Capellera-Garcia,Feiyang Ma,Hieu T. Nim,Mirana Ramialison,Constanze Bonifer,Hanna K. A. Mikkola,Edouard G. Stanley & Andrew G. Elefanty
Nature Biotechnology Published:02 September 2024
DOI:https://doi.org/10.1038/s41587-024-02360-7
Abstract
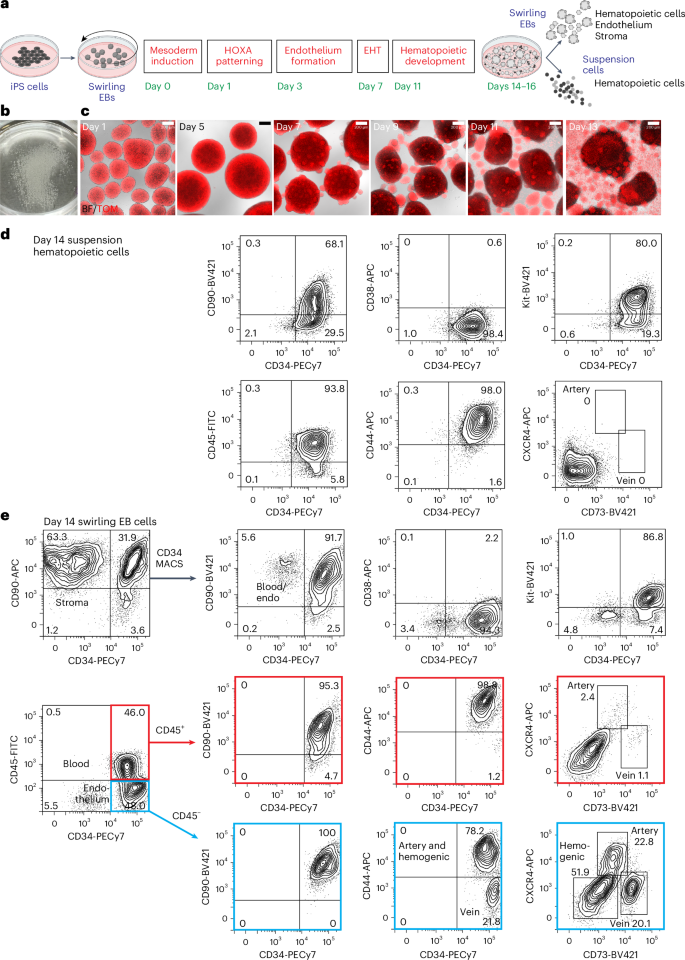
Hematopoietic stem cells (HSCs) derived from human induced pluripotent stem cells (iPS cells) have important biomedical applications. We identified differentiation conditions that generate HSCs defined by robust long-term multilineage engraftment in immune-deficient NOD,B6.Prkdcscid Il2rgtm1Wjl/SzJ KitW41/W41 mice. We guided differentiating iPS cells, as embryoid bodies in a defined culture medium supplemented with retinyl acetate, through HOXA-patterned mesoderm to hemogenic endothelium specified by bone morphogenetic protein 4 and vascular endothelial growth factor (VEGF). Removal of VEGF facilitated an efficient endothelial-to-hematopoietic transition, evidenced by release into the culture medium of CD34+ blood cells, which were cryopreserved. Intravenous transplantation of two million thawed CD34+ cells differentiated from four independent iPS cell lines produced multilineage bone marrow engraftment in 25–50% of immune-deficient recipient mice. These functionally defined, multipotent CD34+ hematopoietic cells, designated iPS cell-derived HSCs (iHSCs), produced levels of engraftment similar to those achieved following umbilical cord blood transplantation. Our study provides a step toward the goal of generating HSCs for clinical translation.
シグナル応答性エンハンサーのゲノムワイドなリレーが造血特異性を駆動する A genome-wide relay of signalling-responsive enhancers drives hematopoietic specification
B. Edginton-White,A. Maytum,S. G. Kellaway,D. K. Goode,P. Keane,I. Pagnuco,S. A. Assi,L. Ames,M. Clarke,P. N. Cockerill,B. Göttgens,J. B. Cazier & C. Bonifer
Nature Communications Published:17 January 2023
DOI:https://doi.org/10.1038/s41467-023-35910-9
Abstract
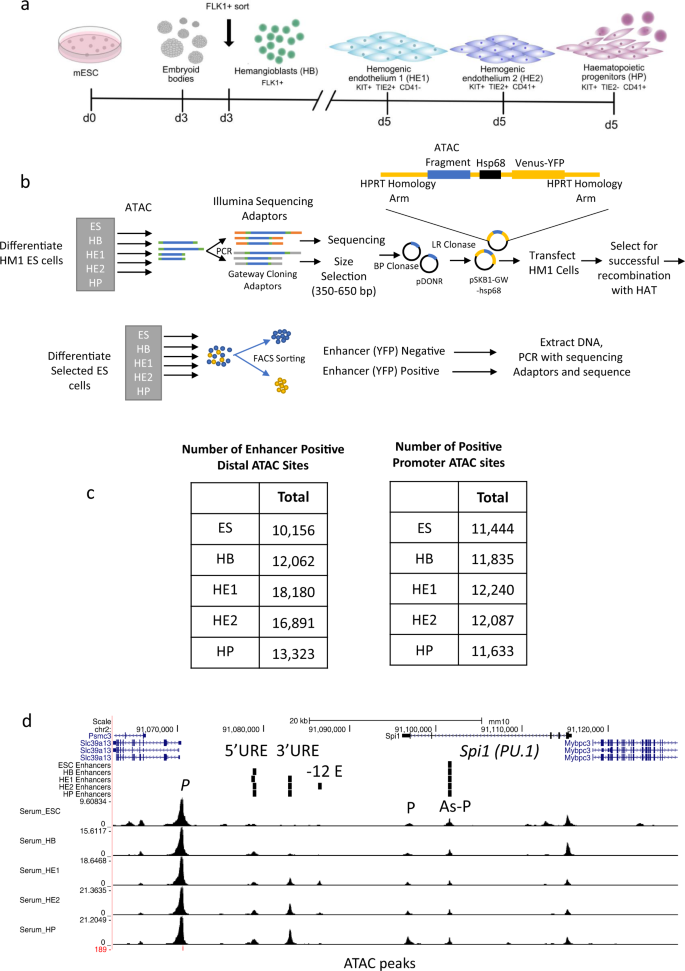
Developmental control of gene expression critically depends on distal cis-regulatory elements including enhancers which interact with promoters to activate gene expression. To date no global experiments have been conducted that identify their cell type and cell stage-specific activity within one developmental pathway and in a chromatin context. Here, we describe a high-throughput method that identifies thousands of differentially active cis-elements able to stimulate a minimal promoter at five stages of hematopoietic progenitor development from embryonic stem (ES) cells, which can be adapted to any ES cell derived cell type. We show that blood cell-specific gene expression is controlled by the concerted action of thousands of differentiation stage-specific sets of cis-elements which respond to cytokine signals terminating at signalling responsive transcription factors. Our work provides an important resource for studies of hematopoietic specification and highlights the mechanisms of how and where extrinsic signals program a cell type-specific chromatin landscape driving hematopoietic differentiation.