2024-09-09 カロリンスカ研究所(KI)
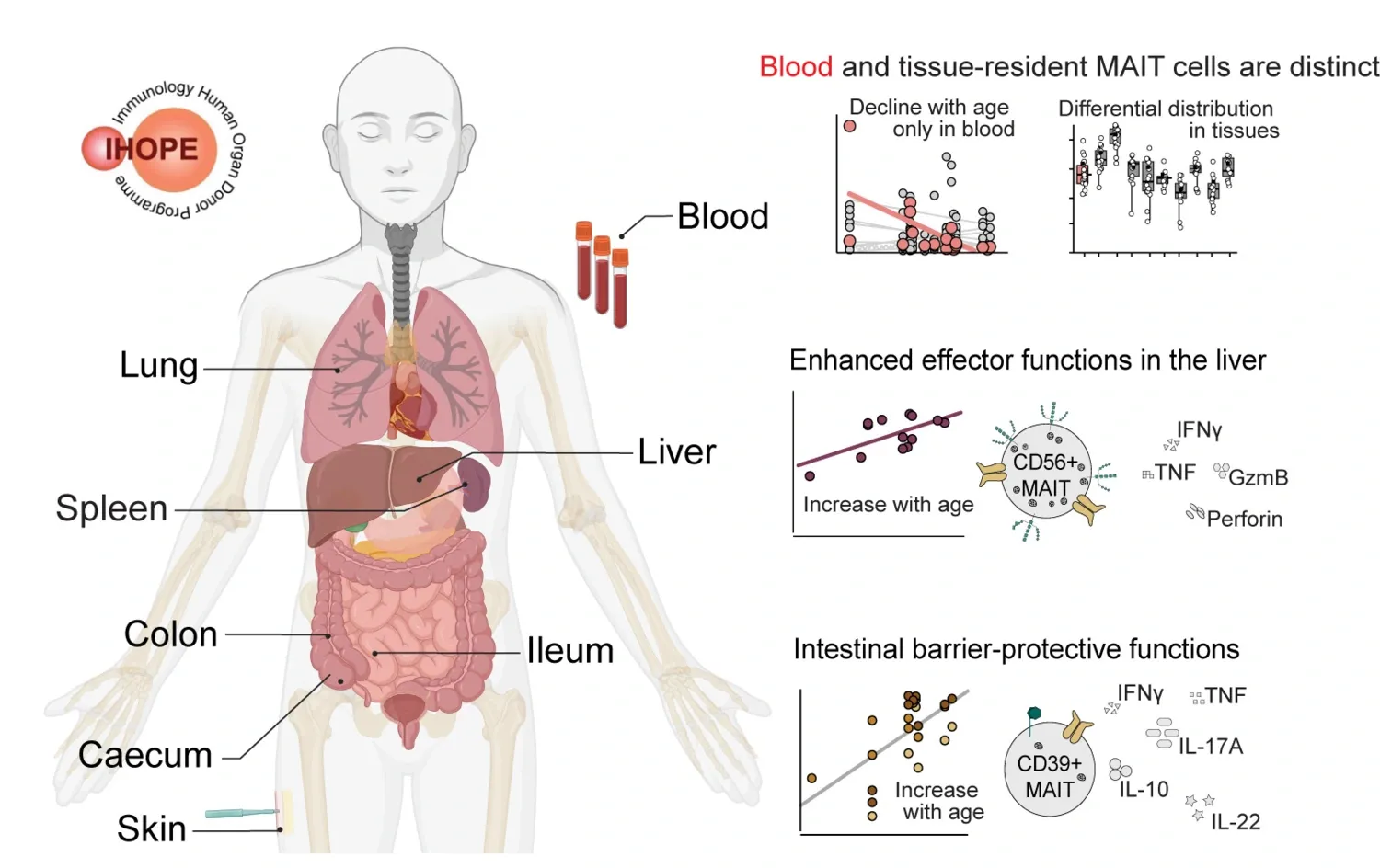 Illustration: Johan Sandberg
Illustration: Johan Sandberg
<関連情報>
- https://news.ki.se/new-study-reveals-specialisation-of-immune-cells-in-different-tissues
- https://www.science.org/doi/10.1126/sciimmunol.adn2362
対になったヒト組織におけるMAIT細胞の不均一性から、異なる制御性と増強されたエフェクター・プロファイルの特殊性が明らかになった MAIT cell heterogeneity across paired human tissues reveals specialization of distinct regulatory and enhanced effector profiles
Tobias Kammann, Curtis Cai, Takuya Sekine, Elli Mouchtaridi, […], and Johan K. Sandberg
Science Immunology Published:6 Sep 2024
DOI:https://doi.org/10.1126/sciimmunol.adn2362
Editor’s summary
Mucosal-associated invariant T (MAIT) cells are a subset of innate-like T cells that recognize microbial riboflavin pathway–derived metabolites. Our understanding of MAIT cell functions in tissue homeostasis and antimicrobial responses has primarily relied on studies of mice and human peripheral blood. Using paired samples from adult human blood and mucosal and lymphoid tissues, Kammann et al. performed a cross-tissue characterization of human MAIT cells. Tissue-specific adaptations included a population of intestinal CD39hi MAIT cells with an immunoregulatory phenotype and liver-enriched CD56+ MAIT cells bearing enhanced effector capacity and innate-like features. Together, these findings highlight the phenotypic and functional specialization of human MAIT cells within specific tissue sites. —Claire Olingy
Abstract
Mucosal-associated invariant T (MAIT) cells are unconventional T cells that recognize microbial riboflavin pathway metabolites presented by evolutionarily conserved MR1 molecules. We explored the human MAIT cell compartment across organ donor–matched blood, barrier, and lymphoid tissues. MAIT cell population size was donor dependent with distinct tissue compartmentalization patterns and adaptations: Intestinal CD103+ resident MAIT cells presented an immunoregulatory CD39highCD27low profile, whereas MAIT cells expressing NCAM1/CD56 dominated in the liver and exhibited enhanced effector capacity with elevated response magnitude and polyfunctionality. Both intestinal CD39high and hepatic CD56+ adaptations accumulated with donor age. CD56+ MAIT cells displayed limited T cell receptor–repertoire breadth, elevated MR1 binding, and a transcriptional profile skewed toward innate activation pathways. Furthermore, CD56 was dynamically up-regulated to a persistent steady-state equilibrium after exposure to antigen or IL-7. In summary, we demonstrate functional heterogeneity and tissue site adaptation in resident MAIT cells across human barrier tissues with distinct regulatory and effector signatures.


