2024-12-18 ゲーテ大学
<関連情報>
- https://aktuelles.uni-frankfurt.de/english/research-into-new-therapies-how-the-bodys-natural-killer-cells-could-fight-leukemia/
- https://www.nature.com/articles/s41467-024-52388-1
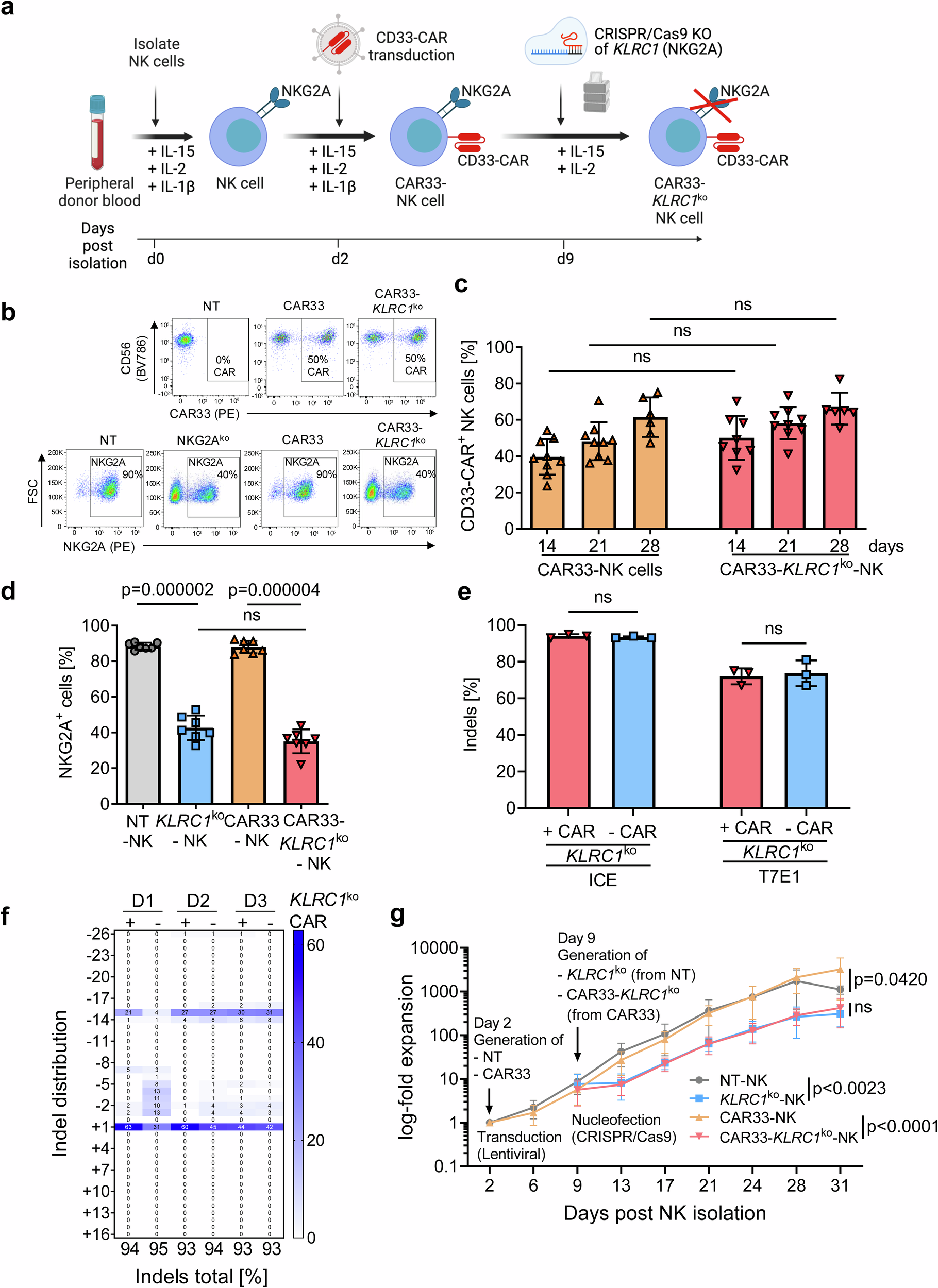
NKG2AのCRISPR/Cas9編集がCD33指向性初代キメラ抗原受容体ナチュラルキラー細胞の有効性を改善する CRISPR/Cas9 editing of NKG2A improves the efficacy of primary CD33-directed chimeric antigen receptor natural killer cells
Tobias Bexte,Nawid Albinger,Ahmad Al Ajami,Philipp Wendel,Leon Buchinger,Alec Gessner,Jamal Alzubi,Vinzenz Särchen,Meike Vogler,Hadeer Mohamed Rasheed,Beate Anahita Jung,Sebastian Wolf,Raj Bhayadia,Thomas Oellerich,Jan-Henning Klusmann,Olaf Penack,Nina Möker,Toni Cathomen,Michael A. Rieger,Katharina Imkeller & Evelyn Ullrich
Nature Communications Published:30 September 2024
DOI:https://doi.org/10.1038/s41467-024-52388-1
Abstract
Chimeric antigen receptor (CAR)-modified natural killer (NK) cells show antileukemic activity against acute myeloid leukemia (AML) in vivo. However, NK cell-mediated tumor killing is often impaired by the interaction between human leukocyte antigen (HLA)-E and the inhibitory receptor, NKG2A. Here, we describe a strategy that overcomes CAR-NK cell inhibition mediated by the HLA-E-NKG2A immune checkpoint. We generate CD33-specific, AML-targeted CAR-NK cells (CAR33) combined with CRISPR/Cas9-based gene disruption of the NKG2A-encoding KLRC1 gene. Using single-cell multi-omics analyses, we identified transcriptional features of activation and maturation in CAR33-KLRC1ko-NK cells, which are preserved following exposure to AML cells. Moreover, CAR33-KLRC1ko-NK cells demonstrate potent antileukemic killing activity against AML cell lines and primary blasts in vitro and in vivo. We thus conclude that NKG2A-deficient CAR-NK cells have the potential to bypass immune suppression in AML.


