2025-03-06 ノースウェスタン大学
<関連情報>
- https://news.northwestern.edu/stories/2025/03/alzheimers-treatment-may-lie-in-the-brain-immune-cells-microglia-amyloid/
- https://www.nature.com/articles/s41591-025-03574-1
免疫化アルツハイマー病患者におけるアミロイドβクリアランスはミクログリア機序によって促進される Microglial mechanisms drive amyloid-β clearance in immunized patients with Alzheimer’s disease
Lynn van Olst,Brooke Simonton,Alex J. Edwards,Anne V. Forsyth,Jake Boles,Pouya Jamshidi,Thomas Watson,Nate Shepard,Talia Krainc,Benney MR Argue,Ziyang Zhang,Joshua Kuruvilla,Lily Camp,Mengwei Li,Hang Xu,Jeanette L. Norman,Joshua Cahan,Robert Vassar,Jinmiao Chen,Rudolph J. Castellani,James AR Nicoll,Delphine Boche & David Gate
Nature Medicine Published:06 March 2025
DOI:https://doi.org/10.1038/s41591-025-03574-1
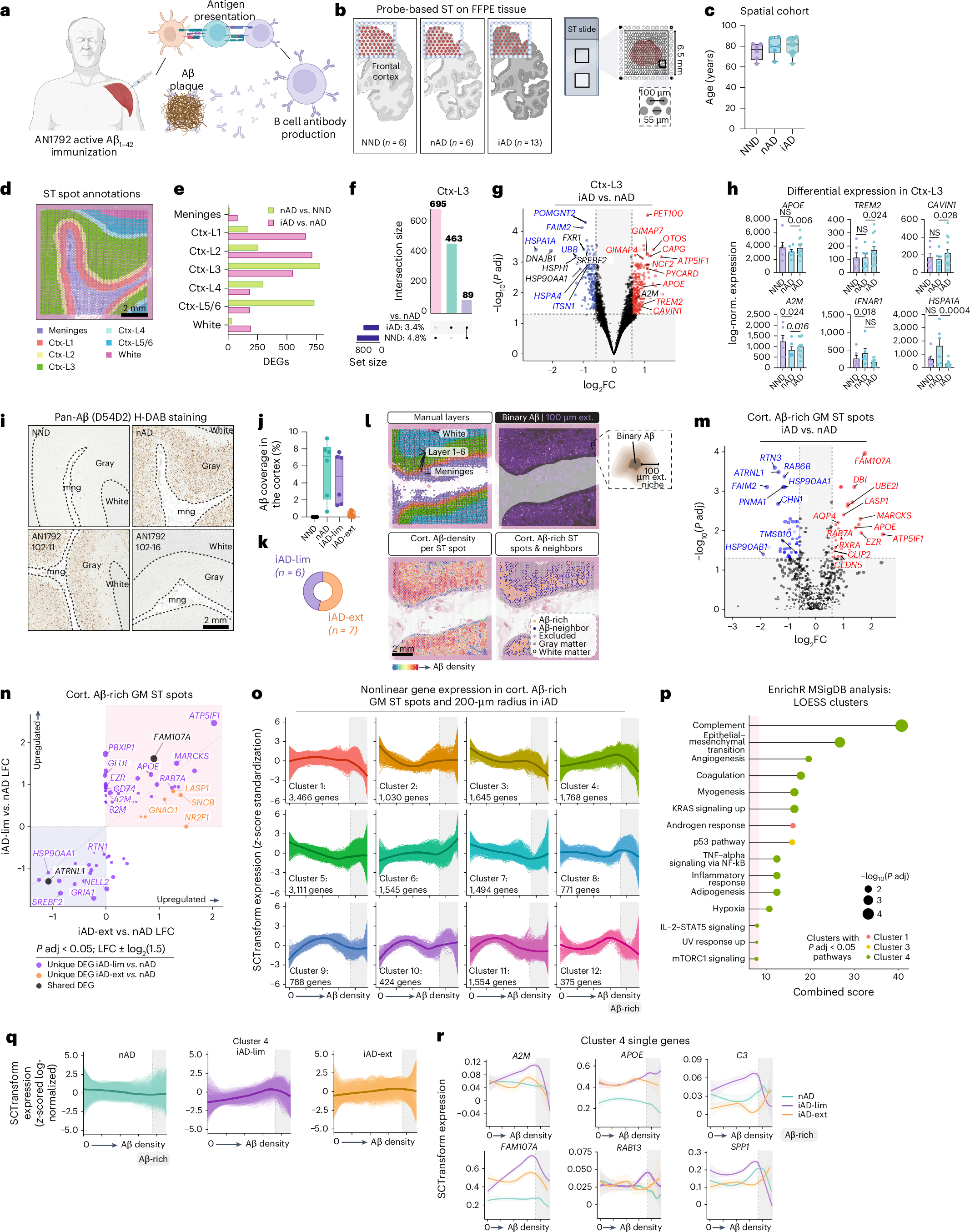
Abstract
Alzheimer’s disease (AD) therapies utilizing amyloid-β (Aβ) immunization have shown potential in clinical trials. Yet, the mechanisms driving Aβ clearance in the immunized AD brain remain unclear. Here, we use spatial transcriptomics to explore the effects of both active and passive Aβ immunization in the AD brain. We compare actively immunized patients with AD with nonimmunized patients with AD and neurologically healthy controls, identifying distinct microglial states associated with Aβ clearance. Using high-resolution spatial transcriptomics alongside single-cell RNA sequencing, we delve deeper into the transcriptional pathways involved in Aβ removal after lecanemab treatment. We uncover spatially distinct microglial responses that vary by brain region. Our analysis reveals upregulation of the triggering receptor expressed on myeloid cells 2 (TREM2) and apolipoprotein E (APOE) in microglia across immunization approaches, which correlate positively with antibody responses and Aβ removal. Furthermore, we show that complement signaling in brain myeloid cells contributes to Aβ clearance after immunization. These findings provide new insights into the transcriptional mechanisms orchestrating Aβ removal and shed light on the role of microglia in immune-mediated Aβ clearance. Importantly, our work uncovers potential molecular targets that could enhance Aβ-targeted immunotherapies, offering new avenues for developing more effective therapeutic strategies to combat AD.