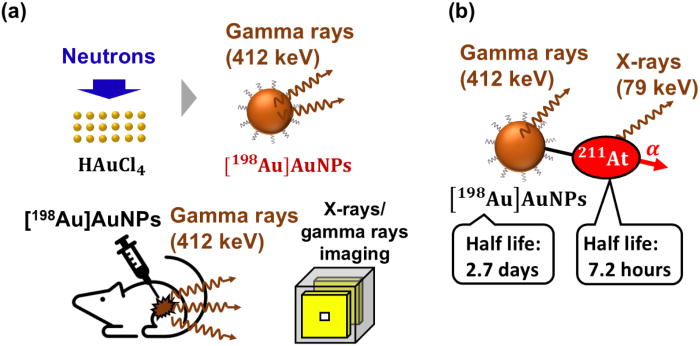
2024-03-12 岐阜大学,国立がん研究センター,科学技術振興機構
<関連情報>
- https://www.ncc.go.jp/jp/information/pr_release/2025/0312/index.html
- https://www.ncc.go.jp/jp/information/pr_release/2025/0312/20250312.pdf
- https://www.nature.com/articles/s41467-025-57617-9
細胞外小胞のレシピエント細胞への取り込みはパラクリン接着シグナルによって促進される Uptake of small extracellular vesicles by recipient cells is facilitated by paracrine adhesion signaling
Koichiro M. Hirosawa,Yusuke Sato,Rinshi S. Kasai,Eriko Yamaguchi,Naoko Komura,Hiromune Ando,Ayuko Hoshino,Yasunari Yokota & Kenichi G. N. Suzuki
Nature Communications Published:12 March 2025
DOI:https://doi.org/10.1038/s41467-025-57617-9
Abstract
Small extracellular vesicles (sEVs) play crucial roles in intercellular communication. However, the internalization of individual sEVs by recipient cells has not been directly observed. Here, we examined these mechanisms using state-of-the-art imaging techniques. Single-molecule imaging shows that tumor-derived sEVs can be classified into several subtypes. Simultaneous single-sEV particle tracking and observation of super-resolution movies of membrane invaginations in living cells reveal that all sEV subtypes are internalized via clathrin-independent endocytosis mediated by galectin-3 and lysosome-associated membrane protein-2C, while some subtypes that recruited raft markers are internalized through caveolae. Integrin β1 and talin-1 accumulate in recipient cell plasma membranes beneath all sEV subtypes. Paracrine, but not autocrine, sEV binding triggers Ca2+ mobilization induced by the activation of Src family kinases and phospholipase Cγ. Subsequent Ca2+-induced activation of calcineurin–dynamin promotes sEV internalization, leading to the recycling pathway. Thus, we clarified the detailed mechanisms of sEV internalization driven by paracrine adhesion signaling.