2025-04-08 パシフィック・ノースウェスト国立研究所(PNNL)
<関連情報>
- https://www.pnnl.gov/news-media/disturbed-sleep-cycle-propels-cyanobacteria-surprising-burst-productivity
- https://microbialcellfactories.biomedcentral.com/articles/10.1186/s12934-025-02665-5
Cyanobacterial circadian regulation enhances bioproduction under subjective nighttime through rewiring of carbon partitioning dynamics, redox balance orchestration, and cell cycle modulation シアノバクテリアの概日制御は、炭素分配ダイナミクスの再配線、酸化還元バランスの調整、細胞周期の調節を通じて、主観的な夜間の生物生産を促進する
Ashley Gilliam,Natalie C. Sadler,Xiaolu Li,Marci Garcia,Zachary Johnson,Marija Veličković,Young-Mo Kim,Song Feng,Wei-Jun Qian,Margaret S. Cheung & Pavlo Bohutskyi
Microbial Cell Factories Published:08 March 2025
DOI:https://doi.org/10.1186/s12934-025-02665-5
Abstract
Background
The industrial feasibility of photosynthetic bioproduction using cyanobacterial platforms remains challenging due to insufficient yields, particularly due to competition between product formation and cellular carbon demands across different temporal phases of growth. This study investigates how circadian clock regulation impacts carbon partitioning between storage, growth, and product synthesis in Synechococcus elongatus PCC 7942, and provides insights that suggest potential strategies for enhanced bioproduction.
Results
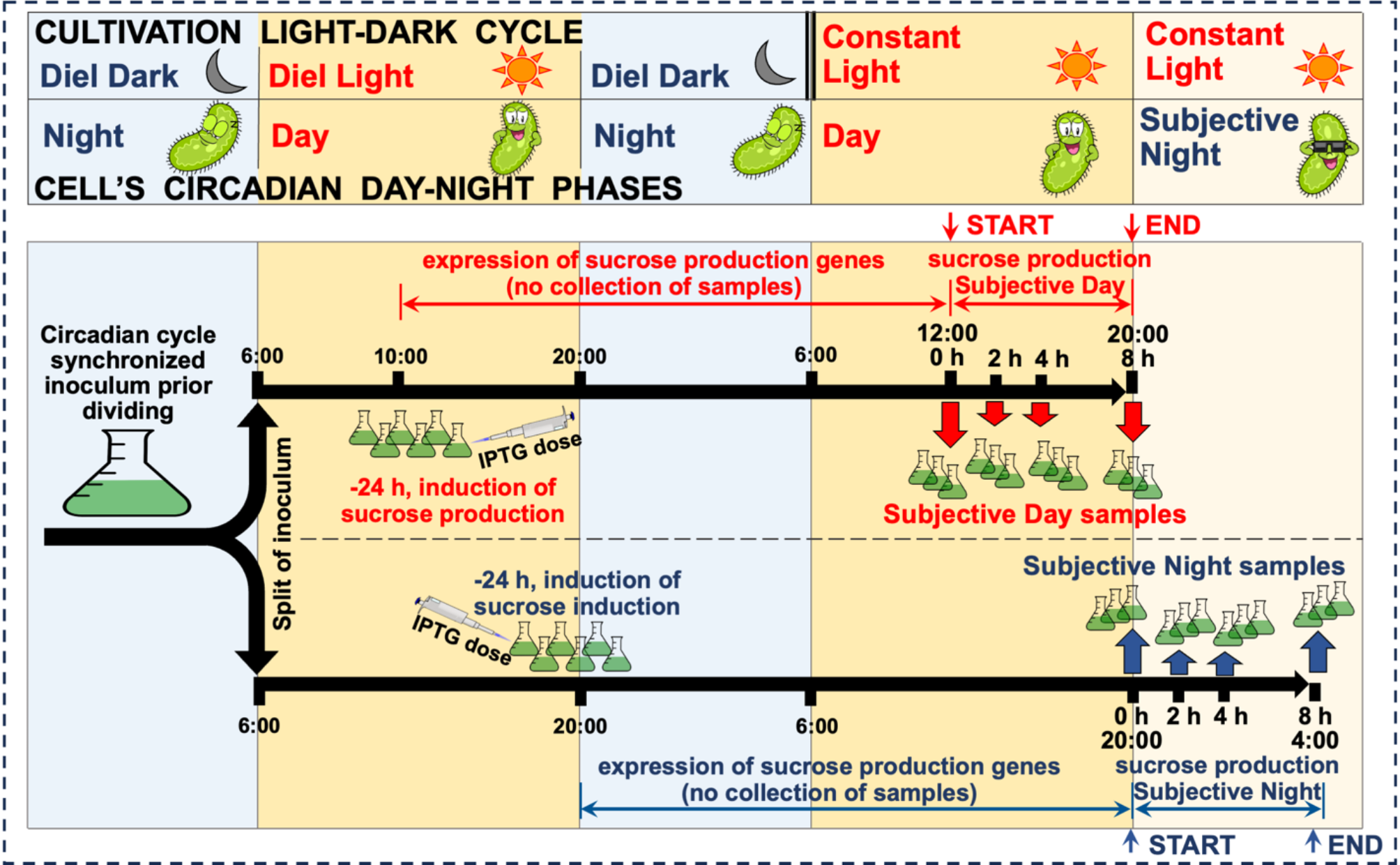
After entrainment to light-dark cycles, PCC 7942 cultures transitioned to constant light revealed distinct temporal patterns in sucrose production, exhibiting three-fold higher productivity during subjective night compared to subjective day despite moderate down-regulation of genes from the photosynthetic apparatus. This enhanced productivity coincided with reduced glycogen accumulation and halted cell division at subjective night time, suggesting temporal separation of competing processes. Transcriptome analysis revealed coordinated circadian clock-driven adjustment of the cell cycle and rewiring of energy and carbon metabolism, with over 300 genes showing differential expression across four time points. The subjective night was characterized by altered expression of cell division-related genes and reduced expression of genes involved in glycogen synthesis, while showing upregulation of glycogen degradation pathways, alternative electron flow components, the pentose phosphate pathway, and oxidative decarboxylation of pyruvate. These molecular changes created favorable conditions for product formation through enhanced availability of major sucrose precursors (glucose-1-phosphate and fructose-6-phosphate) and maintained redox balance through multiple mechanisms.
Conclusions
Our analysis of circadian regulatory rewiring of carbon metabolism and redox balancing suggests two potential approaches that could be developed for improving cyanobacterial bioproduction: leveraging natural circadian rhythms for optimizing cultivation conditions and timing of pathway induction, and engineering strains that mimic circadian-driven metabolic shifts through controlled carbon flux redistribution and redox rebalancing. While these strategies remain to be tested, they could theoretically improve the efficiency of photosynthetic bioproduction by enabling better temporal separation between cell growth, carbon storage accumulation, and product synthesis phases.


