2025-07-02 カリフォルニア大学ロサンゼルス校(UCLA)
Hill Chang and Yifei Miao
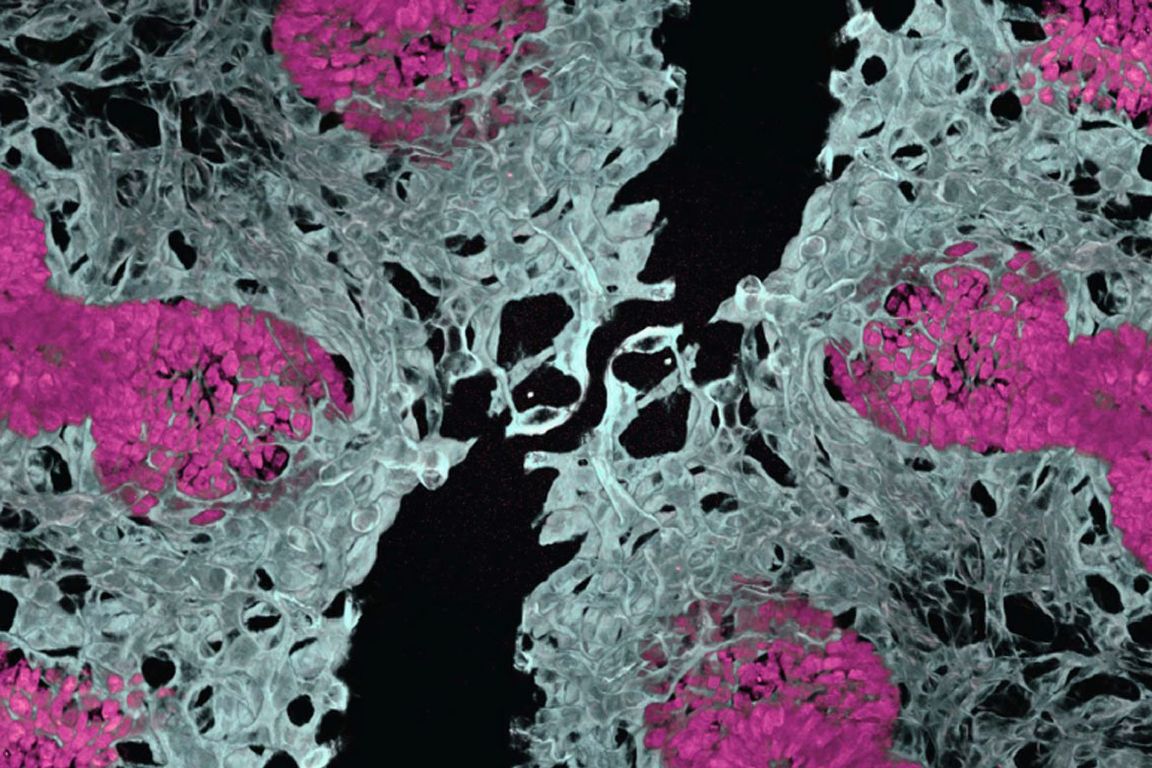
Embryonic mouse lung showing blood vessels (white) and air sacs (pink). This new method to vascularize lung organoids would reduce the reliance on animal models in research.
<関連情報>
- https://newsroom.ucla.edu/stories/mini-lungs-blood-vessels-created-ucla-scientists-pulmonary-vascular-disease
- https://www.cell.com/cell/abstract/S0092-8674(25)00628-2
中胚葉と内胚葉の共発達が肺と腸のオルガノイドにおける器官型血管形成を可能にする Co-development of mesoderm and endoderm enables organotypic vascularization in lung and gut organoids
Yifei Miao ∙ Nicole M. Pek ∙ Cheng Tan ∙ … ∙ Ya-Wen Chen ∙ Minzhe Guo ∙ Mingxia Gu
Cell Published:June 30, 2025
DOI:https://doi.org/10.1016/j.cell.2025.05.041
Highlights
- BMP-mediated mesoderm-endoderm co-differentiation is vital for organotypic vasculature
- Organotypic vasculature supports endoderm organoid development and maturation
- Vascularized organoids resemble human fetal lung and intestine features
- Vascularized organoids model abnormal endothelial-epithelial crosstalk in FOXF1 disease
Summary
The vasculature and mesenchyme exhibit distinct organ-specific characteristics adapted to local physiological needs, shaped by microenvironmental and cell-cell interactions from early development. To recapitulate this entire process, we co-differentiated mesoderm and endoderm within the same spheroid to vascularize lung and intestinal organoids from induced pluripotent stem cells (iPSCs). Bone morphogenetic protein (BMP) signaling fine-tuned the endoderm-to-mesoderm ratio, a critical step in generating appropriate proportions of endothelial and epithelial progenitors with tissue specificity. Single-cell RNA sequencing (scRNA-seq) revealed organ-specific gene signatures of endothelium and mesenchyme and identified key ligands driving endothelial specification. The endothelium exhibited tissue-specific barrier function, enhanced organoid maturation, cellular diversity, and alveolar formation on the engineered lung scaffold. Upon transplantation into mice, the organoid vasculature integrated with the host circulation while preserving organ specificity, further promoting organoid maturation. Leveraging these vascularized organoids, we uncovered abnormal endothelial-epithelial crosstalk in patients with forkhead box F1 (FOXF1) mutations. Multilineage organoids provide an advanced platform to study intricate cell-to-cell communications in human organogenesis and disease.


