2025-08-21 国立がん研究センター
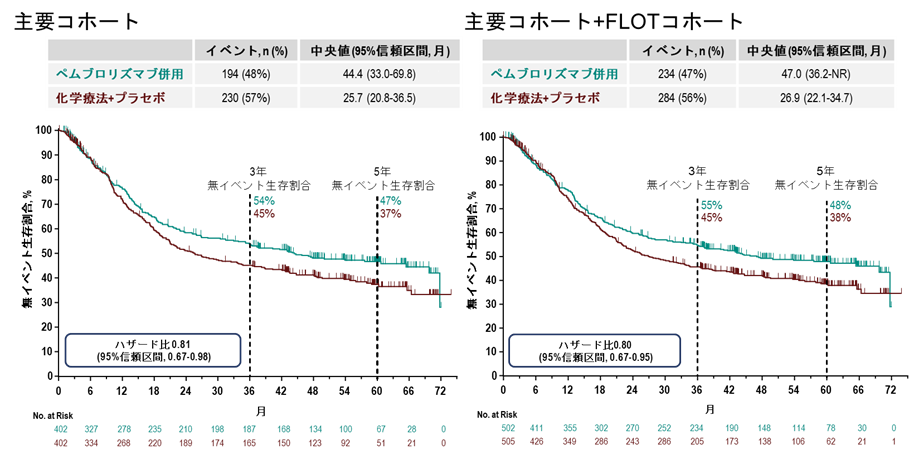
図1. 無イベント生存割合の生存曲線
<関連情報>
- https://www.ncc.go.jp/jp/information/researchtopics/2025/0821/index.html
- https://www.ncc.go.jp/jp/information/researchtopics/2025/0821/20250821.pdf
- https://ascopubs.org/doi/10.1200/JCO-25-00486
局所進行性胃がんおよび胃食道接合部がんにおける術前・術後療法としてのペムブロリズマブ+化学療法と化学療法の比較:ランダム化第III相KEYNOTE-585試験の最終解析 Pembrolizumab Plus Chemotherapy Versus Chemotherapy as Perioperative Therapy in Locally Advanced Gastric and Gastroesophageal Junction Cancer: Final Analysis of the Randomized, Phase III KEYNOTE-585 Study
Kohei Shitara, MD, Sun Young Rha, MD, Lucjan Wyrwicz, MD, Takashi Oshima, MD, Nina Karaseva, MD, Mikhail Osipov, MD, Hisateru Yasui, MD, …
Journal of Clinical Oncology Published:August 19, 2025
DOI:https://doi.org/10.1200/JCO-25-00486
Abstract
We report results of the final analysis of overall survival (OS) and patient-reported outcomes from the phase III KEYNOTE-585 (ClinicalTrials.gov identifier: NCT03221426) study. Participants with previously untreated, locally advanced, resectable gastric and gastroesophageal junction (G/GEJ) cancer were enrolled into the main (n = 804) and fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT; n = 203) cohorts, and randomly assigned 1:1 to neoadjuvant and adjuvant pembrolizumab plus chemotherapy or placebo plus chemotherapy. The primary end points were pathologic complete response (pathCR) by central review, event-free survival (EFS) by investigator, OS, and safety. Patient-reported outcomes was an exploratory end point. After a median follow-up of 59.9 months (range, 39-76), median OS was 71.8 versus 55.7 months (hazard ratio [HR], 0.86 [95% CI, 0.71 to 1.06]) with pembrolizumab plus chemotherapy versus placebo plus chemotherapy in the main cohort. The EFS HR was 0.81 (95% CI, 0.67 to 0.98). Grade ≥3 drug-related adverse event rates were 65% versus 63%. Perioperative pembrolizumab plus chemotherapy did not worsen health-related quality of life versus placebo. Pembrolizumab plus chemotherapy continued to show improved outcomes in pathCR and a trend toward longer EFS versus placebo in the main and main plus FLOT cohorts. Efficacy and safety outcomes with perioperative pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab in participants with untreated, locally advanced resectable G/GEJ cancer were consistent with previous analyses.