2022-09-15 オークリッジ国立研究所(ORNL)
研究チームは、『eLife』誌に発表した論文の中で、ブラジキニン(血管を拡張し透過性を高める化合物)の過剰産生が、肺に過剰な体液がたまる、疲労、吐き気、認知機能の低下といったCOVID-19症状の原因である可能性を予測している。
今回の研究では、計算機による研究をウェットラボで行い、酵素活性と構造的相互作用を確認するための新しいデータセットを作成した。これらの証拠がすべて揃い、これまでの研究で予測されていたことがすべて事実であることが検証された。
<関連情報>
- https://www.ornl.gov/news/lab-experiments-support-covid-19-bradykinin-storm-theory
- https://www.nature.com/articles/s41467-022-32922-9
- https://elifesciences.org/articles/59177
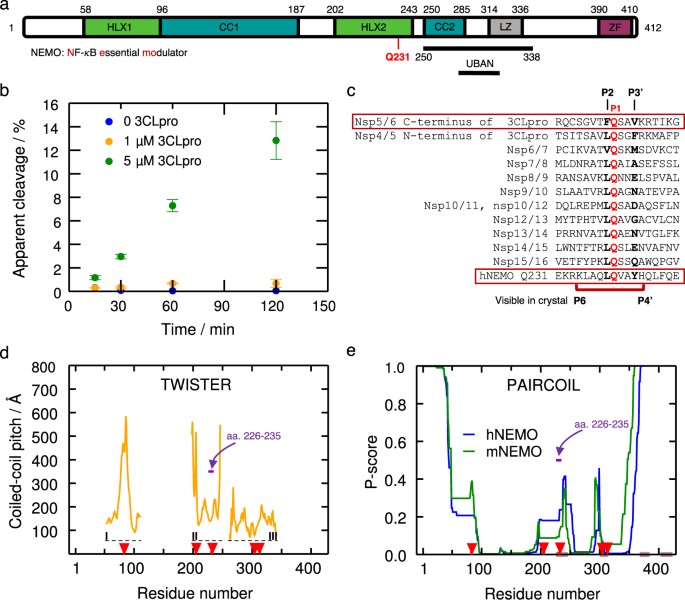
SARS-CoV-2 3CLproによるNEMO切断の構造的・機能的特性評価 Structural and functional characterization of NEMO cleavage by SARS-CoV-2 3CLpro
Mikhail A. Hameedi,Erica T. Prates,Michael R. Garvin,Irimpan I. Mathews,B. Kirtley Amos,Omar Demerdash,Mark Bechthold,Mamta Iyer,Simin Rahighi,Daniel W. Kneller,Andrey Kovalevsky,Stephan Irle,Van-Quan Vuong,Julie C. Mitchell,Audrey Labbe,Stephanie Galanie,Soichi Wakatsuki & Daniel Jacobson
Nature Communications Published:08 September 2022
DOI:https://doi.org/10.1038/s41467-022-32922-9
Abstract
In addition to its essential role in viral polyprotein processing, the SARS-CoV-2 3C-like protease (3CLpro) can cleave human immune signaling proteins, like NF-κB Essential Modulator (NEMO) and deregulate the host immune response. Here, in vitro assays show that SARS-CoV-2 3CLpro cleaves NEMO with fine-tuned efficiency. Analysis of the 2.50 Å resolution crystal structure of 3CLpro C145S bound to NEMO226–234 reveals subsites that tolerate a range of viral and host substrates through main chain hydrogen bonds while also enforcing specificity using side chain hydrogen bonds and hydrophobic contacts. Machine learning- and physics-based computational methods predict that variation in key binding residues of 3CLpro-NEMO helps explain the high fitness of SARS-CoV-2 in humans. We posit that cleavage of NEMO is an important piece of information to be accounted for, in the pathology of COVID-19.
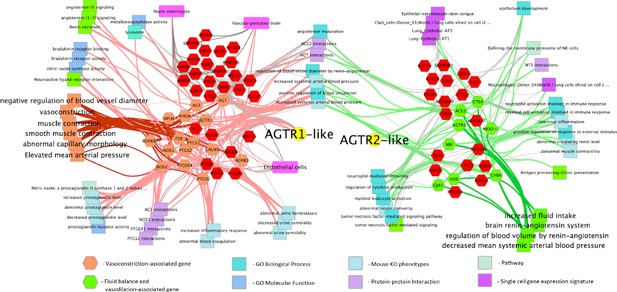
RASを介したブラジキニンストームが関与するCOVID-19のメカニズムモデルおよび治療介入法 A mechanistic model and therapeutic interventions for COVID-19 involving a RAS-mediated bradykinin storm
Michael R Garvin,Christiane Alvarez,J Izaak Miller,Erica T Prates,Angelica M Walker,B Kirtley Amos,Alan E Mast,Amy Justice,Bruce Aronow,Daniel Jacobson
eLife Published:Jul 7, 2020
DOI:https://doi.org/10.7554/eLife.59177
Abstract
Neither the disease mechanism nor treatments for COVID-19 are currently known. Here, we present a novel molecular mechanism for COVID-19 that provides therapeutic intervention points that can be addressed with existing FDA-approved pharmaceuticals. The entry point for the virus is ACE2, which is a component of the counteracting hypotensive axis of RAS. Bradykinin is a potent part of the vasopressor system that induces hypotension and vasodilation and is degraded by ACE and enhanced by the angiotensin1-9 produced by ACE2. Here, we perform a new analysis on gene expression data from cells in bronchoalveolar lavage fluid (BALF) from COVID-19 patients that were used to sequence the virus. Comparison with BALF from controls identifies a critical imbalance in RAS represented by decreased expression of ACE in combination with increases in ACE2, renin, angiotensin, key RAS receptors, kinogen and many kallikrein enzymes that activate it, and both bradykinin receptors. This very atypical pattern of the RAS is predicted to elevate bradykinin levels in multiple tissues and systems that will likely cause increases in vascular dilation, vascular permeability and hypotension. These bradykinin-driven outcomes explain many of the symptoms being observed in COVID-19.