2025-09-03 ペンシルベニア州立大学(PennState)
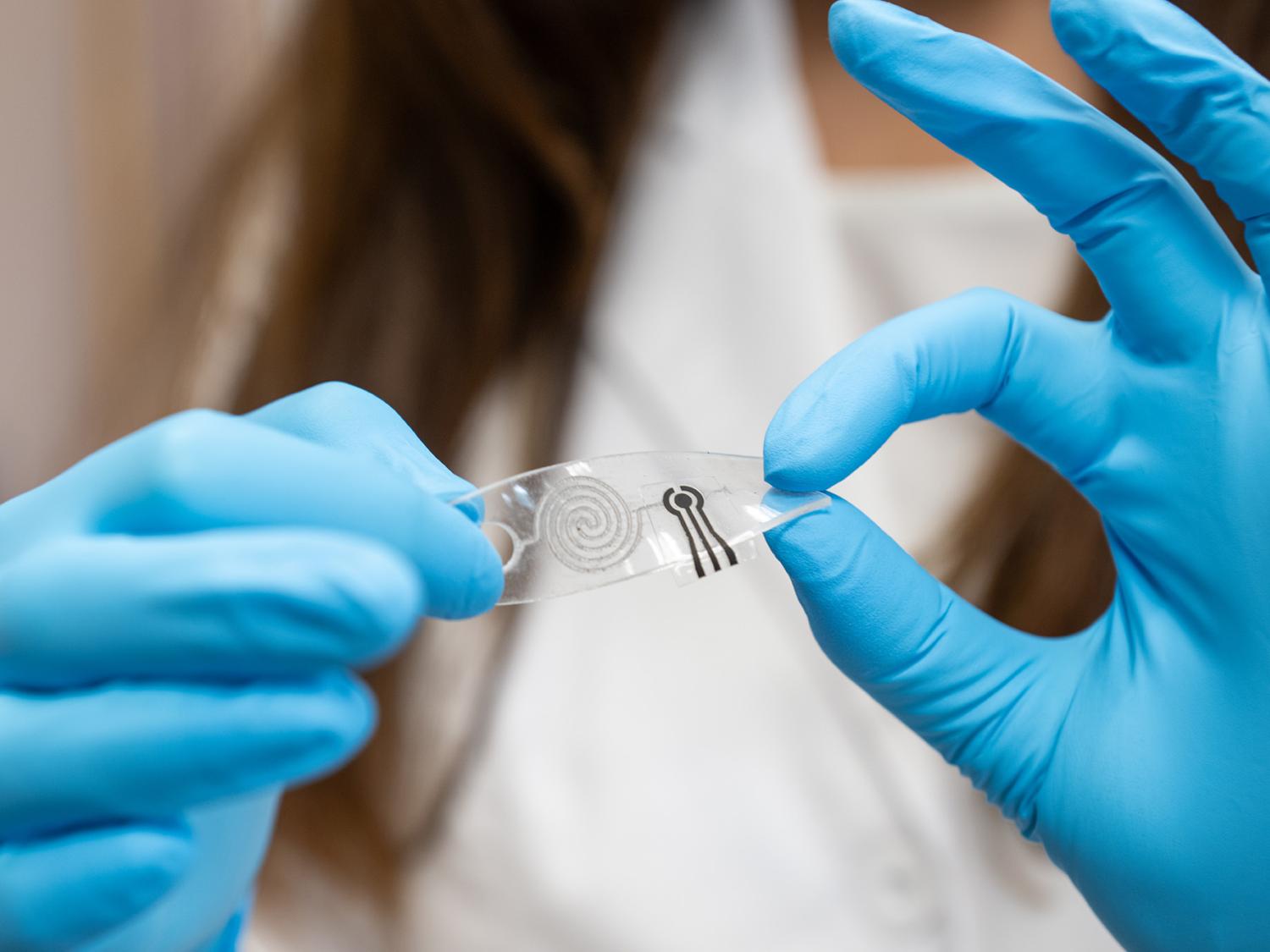
A team at Penn State has developed a novel wearable sensor capable of continuously monitoring low rates of perspiration for the presence of a lactate — a molecule the body uses to break down sugars for energy. Credit: Provided by Farnaz Lorestani. All Rights Reserved.
<関連情報>
- https://www.psu.edu/news/research/story/dont-sweat-it-new-device-detects-sweat-biomarker-minimal-perspiration-rate
- https://onlinelibrary.wiley.com/doi/10.1002/smll.202502655
- https://advanced.onlinelibrary.wiley.com/doi/full/10.1002/adhm.202402489
持続的非侵襲的汗中乳酸検出のための粒状ハイドロゲル搭載ウェアラブル電気化学バイオセンシングプラットフォーム A Granular Hydrogel-Enabled Wearable Electrochemical Biosensing Platform for Continuous Non-Invasive Sweat Lactate Detection
Farnaz Lorestani, Xianzhe Zhang, Zaman Ataie, Alexander Kedzierski, Yushen Liu, Aarón López, Ankan Dutta, Kyle Kacala, Zhenyuan Niu, Amir Sheikhi, Huanyu Cheng
Small Published: 10 June 2025
DOI:https://doi.org/10.1002/smll.202502655
Abstract
Although continuous and non-invasive measurements of sweat biomarkers may provide vital health information, sweat collection often involves intense physical activities or chemical/thermal stimuli. The natural body sweat during endogenous metabolic or stress processes, secreted at much lower rates at rest, may be continuously analyzed using microfluidic devices integrated with hydrophilic rigid fillers; however, the sweat uptake and accumulation in thermoregulatory processes take too long for near-real-time measurements. This work provides an innovative body fluid collection strategy using a granular hydrogel scaffold (GHS), facilitating osmotic and capillary effects to uptake and transfer an ultralow amount of sweat into a microfluidic device at rest. Taken together with a spiral microfluidic channel, the GHS-embedded microfluidics reduce the evaporation of collected sweat and store it in a sensing well for near-real-time measurements. Integrating the sweat-collecting system with an enzymatic gold-graphene nanocomposite-modified laser-induced graphene (LIG) electrode and a LIG-based pH sensor enables the accurate continuous on-body detection of sweat lactate during normal daily activities at a low perspiration rate. The novel combination of a GHS-integrated microfluidic system with a low-cost, flexible, sensitive, and stable LIG-based sensing system provides an accessible technology for sweat-based biosensing during normal daily activities.
ゼラチンメタクリロイル粒状ハイドロゲルスキャフォールドの物理的・生物学的特性を調整するためのマイクロゲル充填の設計 Engineering Microgel Packing to Tailor the Physical and Biological Properties of Gelatin Methacryloyl Granular Hydrogel Scaffolds
Arian Jaberi, Alexander Kedzierski, Sina Kheirabadi, Yerbol Tagay, Zaman Ataie, Saman Zavari, Mohammad Naghashnejad, Olivia Waldron, Daksh Adhikari, Gerald Lester …
Advanced Healthcare Materials Published: 17 August 2024
DOI:https://doi.org/10.1002/adhm.202402489
Abstract
Granular hydrogel scaffolds (GHS) are fabricated via placing hydrogel microparticles (HMP) in close contact (packing), followed by physical and/or chemical interparticle bond formation. Gelatin methacryloyl (GelMA) GHS have recently emerged as a promising platform for biomedical applications; however, little is known about how the packing of building blocks, physically crosslinked soft GelMA HMP, affects the physical (pore microarchitecture and mechanical/rheological properties) and biological (in vitro and in vivo) attributes of GHS. Here, the GHS pore microarchitecture is engineered via the external (centrifugal) force-induced packing and deformation of GelMA HMP to regulate GHS mechanical and rheological properties, as well as biological responses in vitro and in vivo. Increasing the magnitude and duration of centrifugal force increases the HMP deformation/packing, decreases GHS void fraction and median pore diameter, and increases GHS compressive and storage moduli. MDA-MB-231 human triple negative breast adenocarcinoma cells spread and flatten on the GelMA HMP surface in loosely packed GHS, whereas they adopt an elongated morphology in highly packed GHS as a result of spatial confinement. Via culturing untreated or blebbistatin-treated cells in GHS, the effect of non-muscle myosin II-driven contractility on cell morphology is shown. In vivo subcutaneous implantation in mice confirms a significantly higher endothelial, fibroblast, and macrophage cell infiltration within the GHS with a lower packing density, which is in accordance with the in vitro cell migration outcome. These results indicate that the packing state of GelMA GHS may enable the engineering of cell response in vitro and tissue response in vivo. This research is a fundamental step forward in standardizing and engineering GelMA GHS microarchitecture for tissue engineering and regeneration.


